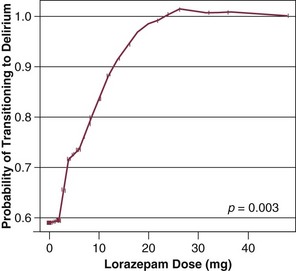
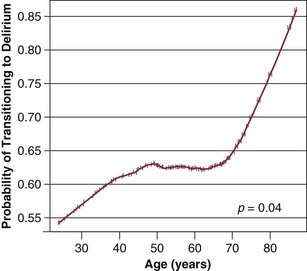
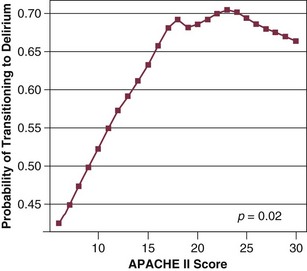
72 ACUTE BRAIN DYSFUNCTION OR DELIRIUM SLEEP DISRUPTION IN THE CRITICALLY ILL PATIENT Definition and Diagnostic Criteria Prevalence of ICU-Related Posttraumatic Stress Disorder Risk Factors for Posttraumatic Stress Disorder Conceptual Explanations for Posttraumatic Stress Disorder After Critical Illness LONG-TERM COGNITIVE IMPAIRMENT AFTER CRITICAL ILLNESS Having acknowledged the preceding realities of the states of survival of critical illness, it is also true that advances in clinical medicine have resulted in dramatic improvements in survival in a host of medical conditions, such as acute respiratory distress syndrome (ARDS) and sepsis. In general, 1- to 3-month mortality rates for both of these common ICU admission diagnoses in most cohorts is now in the 20% to 35% range, whereas in the 1990s these mortality rates were in the 40% to 60% range. As a result of the increased confidence in survival, both the lay public and health care professionals are now increasingly focused on preservation of cognitive abilities, prevention of functional decline, and the quality of life among patients who survive critical illness.1–5 The potential of being left cognitively impaired is a major determinant of patients’ treatment preferences at the end of life, with 9 of every 10 patients preferring death to severe cognitive impairment.6 In support of this reality, in a report from the international “Surviving Intensive Care” 2002 Roundtable Conference held in Brussels,7 the need for future investigations in neurocognitive abnormalities among survivors of intensive care received the strongest recommendation from the international panel of experts. Physicians and health care providers in ICUs are accustomed to recognizing multiple organ dysfunction syndrome (MODS),8–11 with therapy focused on the causes and treatment of respiratory, cardiovascular, renal, and hepatic dysfunction. There are increasing data on the syndrome of brain dysfunction during and following the ICU. This chapter focuses on the cognitive and mental health disturbances of critical illness, with emphasis on delirium and sleep disturbances during critical illness, as well as a brief discussion of PTSD, long-term cognitive impairment (LTCI), and depression after critical illness. Delirium is defined by American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 4th revised edition (DSM-IV),12 as a disturbance of consciousness with the cornerstone component of inattention13 being the pivotal feature of the diagnosis. This alarmingly common form of brain dysfunction often develops acutely (hours to days) in critically ill patients and fluctuates over time. Many different terms have been used to describe this spectrum of cognitive impairment in critically ill patients, including ICU psychosis, ICU syndrome, acute confusional state, septic encephalopathy, and acute brain failure.14–16 The current consensus of many authorities is to use the unifying term delirium and subcategorize according to the level of alertness (hyperactive, hypoactive, or mixed).17 We will take this approach in this chapter. Although the prevalence of delirium in medical ICU cohort studies has been reported to be between 20% and 80%,18–20 more general ranges are practical depending on the severity of illness and the delirium detection instrument used, such as 40% to 60% in nonventilated and 60% to 80% in ventilated ICU patients.21 Unfortunately, because delirium is usually “quietly” manifested by negative symptoms, it remains unrecognized by the clinician in a majority of the patients experiencing this complication,22,23 and it may be incorrectly attributed to dementia, depression, or just an “expected” occurrence in the critically ill, elderly patient.22 Peterson and associates24 reported on delirium subtypes from a cohort of ventilated and nonventilated ICU patients in whom delirium was monitored. They found the rates of these subtypes in the ICU to be 1.6% for hyperactive, 43.5% for hypoactive, and 54.1% for mixed.25 Hyperactive delirium, which is rare in the pure form and associated with a better overall prognosis,26 is characterized by agitation, restlessness, attempting to remove catheters or tubes, hitting, biting, and emotional lability.17,27 This subtype was in the past referred to by the misnomer “ICU psychosis.” Hypoactive delirium, on the other hand, is very common and in many circumstances actually more deleterious for the patient in the long run.26 Unfortunately, it remains unrecognized in 66% to 84% of patients, whether being treated in the ICU, hospital ward, or emergency department.23,28–30 This delirium subtype is characterized by withdrawal, flat affect, apathy, lethargy, and decreased responsiveness.26,31,32 Some authorities continue to refer to the hypoactive delirium as “encephalopathy” and the hyperactive subtype as “delirium or ICU psychosis.” Because of the fluctuating nature of delirium, patients may present with a mixed clinical picture or may sequentially experience both of these subtypes. Many critical care providers would have the incorrect impression that hyperactive delirium is far more common, perhaps because affected patients attract attention because of their immediate threat to self and others. Unless routine monitoring for delirium is implemented as part of an overall ICU bundle such as the ABCDEs (spontaneous Awakening trials, spontaneous Breathing trials, Coordination of care and Choice of sedative, Delirium monitoring and management, and Early mobility),33,34 the majority of delirium episodes, especially the “quiet” (hypoactive) form, will be missed. In non-ICU populations, the development of delirium in the hospital is associated with an in-hospital mortality rate of 25% to 33%, prolonged hospital stay, and three times greater likelihood of discharge to a nursing home.35–37 In a three-site study of non-ICU medical patients, delirium was found to be an independent predictor of the combined outcome of death or nursing home placement.38 McCusker and colleagues39 found a 2.11 adjusted hazard ratio for dying in association with the development of delirium. This mortality rate increase has now been shown to be independent of dementia status.40 Furthermore, three prospective studies have found that delirium was associated with a higher risk for dementia during the 2 to 3 years after hospitalization in non-ICU patient populations.41–43 Among medical ICU patients, delirium has been shown to be a strong predictor of longer duration of mechanical ventilation, longer length of ICU stay, higher costs, prolonged neuropsychological dysfunction, and even death.44–47 The development of delirium was associated with a threefold increase in risk of death after data were controlled for preexisting comorbid conditions, severity of illness, coma, and the use of sedative and analgesic medications.44 Three separate cohort studies have shown that the duration of delirium (in a dose-dependent sort of relationship) is an independent predictor of increased subsequent death, with the most commonly cited finding being that every day a patient spends in ICU delirium portends a 10% higher risk of death even after adjusting for relevant covariates such as severity of illness, age, psychoactive medication use, and coma.44,48,49 In addition, it is now shown that the duration of delirium is an independent predictor of LTCI in general medical and surgical ICU patients who in the past were not suspected of having long-term dementia-like abnormalities.50 From a neuroscience perspective, delirium is thought to be related to imbalances in the synthesis, release, and inactivation of neurotransmitters modulating the control of cognitive function, behavior, and mood.26,32 Three of the neurotransmitter systems involved in the pathophysiology of delirium are dopamine, γ-aminobutyric acid (GABA), and acetylcholine.15,51,52 Whereas dopamine increases excitability of neurons, GABA and acetylcholine decrease neuronal excitability.15 An imbalance in one or more of these neurotransmitters results in neuronal instability and unpredictable neurotransmission. In general, an excess of dopamine and depletion of acetylcholine are two major physiologic problems believed to be central to delirium.53 Other neurotransmitter systems thought to be involved in the development of delirium are serotonin imbalance, endorphin hyperfunction, and increased central noradrenergic activity.26,51 Other factors thought to be mechanistically deliriogenic in ICU patients are inflammatory abnormalities induced by endotoxins and cytokines.54–57 The inflammatory mediators produced in sepsis, such as tumor necrosis factor-α (TNF-α), interleukin 1, and other cytokines and chemokines, initiate a cascade that leads to endothelial damage, thrombin formation, and microvascular compromise.58 Animal models show that these inflammatory mediators cross the blood-brain barrier,59 increase vascular permeability in the brain,60 and result in electroencephalography (EEG) changes consistent with those seen in septic patients with delirium.61,62 Release of inflammatory mediators may occur from (1) decreased cerebral blood flow, a result of the formation of microaggregates of fibrin, platelets, neutrophils, and erythrocytes in the cerebral microvasculature, (2) cerebral vasoconstriction occurring in response to (α1-adrenergic receptor activity63; or (3) interference with neurotransmitter synthesis and neurotransmission.64 One hypothesis attempts to explain acute delirium as a behavioral manifestation of a “widespread reduction of cerebral oxidative metabolism resulting in an imbalance of neurotransmission.”65 On the basis of a series of investigations in which they evaluated delirious patients using EEG, Engel and Romano66 postulated that delirium is a state of “cerebral insufficiency,” that is, a global failure of cerebral oxidative metabolism. Their work showed that delirium is associated with diffuse slowing on EEG, a finding believed to represent a reduction in brain metabolism. Blass and colleagues67 offered a possible link between the state of cerebral insufficiency proposed by Engel and Romano66 and the hypothesis of cholinergic blockade by suggesting that impaired oxidative metabolism in the brain results in a cholinergic deficiency. The finding that hypoxia impairs acetylcholine synthesis supports this hypothesis.68 This reduction in cholinergic function leads to an increase in the level of glutamate, dopamine, and norepinephrine in the brain. Additionally, serotonin and GABA levels are reduced; all of these changes contribute to delirium. Changes in the plasma levels of various amino acid precursors of cerebral neurotransmitters may affect their function, thus contributing to the development of delirium.69 The amino acid entry into the brain is regulated by a sodium-independent large neutral amino acid transporter type 1 (LAT1).70 The essential amino acid tryptophan, which is the precursor for serotonin, competes with several large neutral amino acids (LNAAs), such as tyrosine, phenylalanine, valine, leucine, and isoleucine, for transport across the blood-brain barrier via the LAT1 transporter.70 This competition determines its uptake into the brain.70 Recently, alterations in plasma tryptophan and tyrosine levels were identified as independent risk factors for the transition to clinical delirium.71 Increased activation of the kynurenine pathway, which is involved in the metabolism of tryptophan and the formation of neurotoxins, has also been linked to fewer days alive and without acute brain dysfunction in critically ill patients.72 Another amino acid that may play an important role in the pathogenesis of delirium is phenylalanine.65 Like tryptophan, phenylalanine competes with the other LNAAs (tyrosine, tryptophan, valine, leucine, and isoleucine) for transport across the blood-brain barrier. An increase in the cerebral uptake of tyrosine and phenylalanine compared with the other LNAAs leads to greater availability of precursors for both dopamine and norepinephrine, two neurotransmitters that have been implicated in the pathogenesis of delirium.65 It is important to realize that although delirium may occur as a result of perturbations in other organ systems, the brain responds to systemic infections and injury with an inflammatory response of its own that also involves cytokine production, cell infiltration, and tissue damage.73,74 Reports indicate that local inflammation in the brain and subsequent activation of these central nervous system immune responses are accompanied by manifestations of systemic inflammation,52,75,76 including production of large amounts of peripherally produced TNF-α, interleukin 10, and interferon-γ.73,77–79 Thus, it is postulated that the brain can become an engine of inflammation, driving the development, resolution, or both, of MODS. Van Gool and associates propose that this self-propelling inflammation, in the context of microglial activation, could help explain the association between delirium and LTCI.80 Surprisingly, van Gool points out that animal models have shown activated microglia can remain primed for months following injury, allowing ample time for ongoing injury long beyond the period of an ICU stay. This factors into hypothesis generation when designing neuroimaging studies in ICU survivors and will serve as fascinating topics for future study. Although non-ICU cohort studies have identified numerous risk factors for the development of delirium,37 only a few studies have examined these factors in the ICU population. Baseline risk factors that predispose patients to a greater degree of vulnerability for delirium include Alzheimer’s disease, chronic illness, advanced age, and depression.15,81,82 Dubois and coworkers83 found that preexisting hypertension and smoking (presumably because of relative hypoperfusion and nicotine withdrawal, respectively) were significantly associated with the development of ICU delirium. Another investigation reported that preexisting dementia was a significant risk factor for the development of delirium in the post-ICU period.19 A new area of investigation is genetic predisposition to delirium, as the apolipoprotein E4 polymorphism was found to predict delirium that lasted nearly twice as long as patients without the polymorphism.84 Further studies have continued to suggest this association.85 Precipitating and iatrogenic risk factors represent areas of potential modification and, thus, intervention for delirium prevention and treatment. Precipitating factors are hypoxia, metabolic disturbances, electrolyte imbalances, withdrawal syndromes, acute infection (systemic and intracranial), seizures, dehydration, hyperthermia, sleep deprivation, head trauma, vascular disorders, and intracranial space-occupying lesions.15,81,82 In practical terms, the risk factors for delirium can be divided into the following categories: (1) host factors, (2) the acute illness itself, and (3) iatrogenic or environmental factors (Box 72.1). Although delirium may be a function of patients’ specific underlying illness, it may also be due to medical management issues and thus may have preventable causes. Of these risk factors, sedative and analgesic medications and sleep deprivation appear to be the leading iatrogenic and hence possibly preventable risk factors for delirium. There are conflicting data on the association of anticholinergics, corticosteroids, histamine H2 antagonists, and anticonvulsants with the development of delirium in ICU patients.22,86–88 Sedative and analgesic medications are routinely administered to patients undergoing mechanical ventilation, in accordance with widely recognized clinical practice guidelines by the Society of Critical Care Medicine (SCCM),89 in order to reduce pain and anxiety. Investigations have shown that continuous intravenous sedation is associated with prolonged mechanical ventilation and greater morbidity.90 Similarly, associations between psychoactive medications and worsening cognitive outcomes have been reported in postoperative patients. Marcantonio and colleagues,91 studying postoperative patients in whom delirium developed, found an association between use of benzodiazepines and meperidine and the occurrence of delirium. Dubois and coworkers83 have shown that opiates (morphine and meperidine) administered either intravenously or via an epidural catheter may be associated with the development of delirium in medical/surgical ICU patients. Studies such as these have generated concern about whether such drugs were actually responsible for the development of delirium or were given as a result of delirium. Our group has studied this temporal relationship between the administration of sedatives and analgesics and delirium.92 To do so, one must make repeated cognitive assessments and must be able to assess the risk factors a patient is exposed to in between these assessments in order to study which of these factors is associated with a transition or a change in cognitive status to or from normal, delirium, or coma. In our study, lorazepam was found to be an independent risk factor for daily transition to delirium, and fentanyl, morphine, and propofol were associated with higher but not statistically significant odds ratios for such transition (Fig. 72.1).92 Increasing age and APACHE II (Acute Physiology and Chronic Health Evaluation II) scores were also independent predictors of transitioning to delirium (Figs. 72.2 and 72.3).92 Similar associations between another benzodiazepine, midazolam, and transition to delirium have been found in another study conducted in our trauma and surgical ICU patients.21 At this time it is not clear whether this association between benzodiazepines, and possibly opioids, and delirium is related to the pharmacokinetic properties of the agents or the pharmacodynamics of the drug. Benzodiazepines and propofol have high affinity for the GABA receptor in the central nervous system.93 This GABA-mimetic effect can alter levels of numerous neurotransmitters believed to be deliriogenic.64,94 Novel sedative agents that are GABA receptor-sparing (such as the α2-agonists) may help reduce some of the cognitive dysfunction seen in ICU patients. It is important to note that the data for opioids and delirium are not as consistent as those for the benzodiazepines. Although meperidine has been associated with delirium in most of the published studies, evidence for both fentanyl and morphine has been less convincing.83,91,95 Morrison and colleagues95 conducted a prospective observational trial in patients who had undergone hip surgery and found that patients whose pain was well controlled with morphine were less likely to demonstrate delirium than those who received other opioids. These investigations point to the importance of the judicious use of psychoactive medications, with a focus on adequate analgesia.96 One hopes that ongoing randomized controlled trials will pave the way to the development of sedation and analgesic guidelines for the prevention or reduction of the occurrence of delirium due to the administration of these psychoactive drugs. The development of tools such as the Intensive Care Delirium Screening Checklist (ICDSC)18 and the Confusion Assessment Method for the ICU (CAM-ICU)20 have allowed for the rapid diagnosis of delirium in patients by nonpsychiatric physicians and other health care personnel even while such patients are mechanically ventilated. The SCCM has proposed guidelines for more routine and more diligent monitoring of delirium using reliable and validated scales.89 Diagnosis of delirium is a two-step process. Level of arousal is first measured with the use of a standardized sedation scale, like the Richmond Agitation-Sedation Scale (RASS) (Fig. 72.4).97,98 This is a 10-point scale with scores ranging from +4 to –5, score of 0 denoting a calm and alert patient. Positive RASS scores denote positive or aggressive symptomatology ranging from +1 (mild restlessness) to +4 (dangerous agitation). The negative RASS scores differentiate between response to verbal commands (scores –1 to –3) and physical stimulus (scores –4 and –5). If the patient’s RASS score is –4 or –5 or not arousable by verbal commands, no further evaluation for delirium is performed, because the patient is comatose and is unable to be assessed for delirium. For patients who are arousable (RASS scores of –3 and higher), delirium can be assessed with the ICDSC18 or by the CAM-ICU.20 The ICDSC assesses eight features of delirium: altered level of consciousness, inattention, disorientation, hallucinations, psychomotor agitation/retardation, inappropriate mood/speech, sleep/wake cycle disturbance, and symptom fluctuation. The sensitivity and specificity of this tool are 99% and 64%, respectively.18 The CAM-ICU, which can be performed in about 60 to 90 seconds,99 comprises four features that assess the following: acute change or fluctuation of mental status (feature 1), inattention (feature 2), disorganized thinking (feature 3), and an altered level of consciousness (feature 4). Figure 72.4 Richmond Agitation-Sedation Scale (RASS) and the Confusion Assessment Method for the ICU (CAM-ICU). This sedation scale and delirium instrument can be used together as a two-step approach to assess consciousness and diagnose delirium. Patients are considered to have delirium if they have RASS scores of –3 and higher and are assessed by CAM-ICU as “positive” by having both features 1 and 2 and either feature 3 or feature 4. (Data from references 19, 85, 86, and 148 [available online].) To be diagnosed as delirious, a patient must have a RASS score of –3 or higher, with an acute change or fluctuation in mental status (feature 1), accompanied by inattention (feature 2) and either disorganized thinking (feature 3) or an altered level of consciousness (feature 4). A complete description of the CAM-ICU as well as training materials, including translations and clinical vignettes, can be found at our website (www.icudelirium.org). Recently, a meta-analysis evaluated the accuracy of the CAM-ICU and the ICDSC for the diagnosis of ICU delirium.100 This investigation included nine studies evaluating the CAM-ICU (N = 969 total patients) and four studies evaluating the ICDSC (N = 361). The authors calculated the pooled sensitivity of the CAM-ICU to be 80% (95% confidence interval [CI]: 77.1-82.6%) and specificity of 95.9% (95% CI: 94.8-96.8%). The pooled sensitivity and specificity of the ICDSC were 74% (95% CI: 65.3-81.5%) and 81.9% (95% CI: 76.7-86.4%). They concluded that both delirium scales can be used as a screening tool, and that the CAM-ICU was an excellent diagnostic tool and the ICDSC had moderate sensitivity and good specificity.100 In a trial of 852 general medical patients older than 70 years,101 implementation of strategies for primary prevention of delirium resulted in a 40% reduction in the odds for development of delirium (15% in control subjects vs. 9.9% in the intervention patients). The protocol focused on optimization of risk factors via the following methods: repeated reorientation of the patient by trained volunteers and nurses, provision of cognitively stimulating activities for the patient three times per day, a nonpharmacologic sleep protocol to enhance normalization of sleep/wake cycles, early mobilization activities and range-of-motion exercises, timely removal of catheters and physical restraints, institution of the use of eyeglasses and magnifying lenses, use of hearing aids and earwax disimpaction, and early correction of dehydration.101 Unfortunately, this intervention did not show sustained benefit during the 6 months of follow-up.102 Later studies of delirium were unable to reproduce this success in reducing the incidence of delirium.103,104 Milisen and associates105 and Lundstrom and coworkers,106 who studied the implementation of multifactorial and multidisciplinary educational strategies, reported decreases in the duration and severity of delirium in patients cared for by staff who had received delirium-specific education.105,106 Milisen and associates105 measured the impact on delirium of implementing a nurse-led intervention program that involved delirium education for the nursing staff, systematic cognitive screening, availability of consultative services from a delirium resource nurse, and scheduled pain protocol on delirium. These researchers reported that although the program had no effect on the incidence of delirium, the duration and severity of delirium were significantly lower.105 Lundstrom and coworkers106 reported that in patients on a ward where the staff participated in specific educational activities focused on delirium and where the bedside nursing care was reorganized to provide more continuity of patient-centered care, duration of delirium and hospital stay were shorter and mortality rate was lower than in control patients. Cole and colleagues104 found no difference in delirium rates between patients cared for by an intervention nurse and patients who received standard care. All of these nonpharmacologic “protocolization of care” studies focused on non-ICU patient populations. Clearly such investigations must be designed and conducted in the ICU (rather than simply extrapolated from non-ICU studies). Although primary prevention of delirium is preferred, some delirium is inevitable in the ICU. In patients exhibiting delirium, the basic tenets of patient management, such as restoration of sleep/wake cycles, timely removal of catheters, early mobilization, minimization of unnecessary noise/stimuli, and frequent reorientation, should be applied liberally. Additionally, it should be emphasized that although sedative and analgesic agents have a very important role in patient comfort, health care professionals must also strive to achieve the right balance of administering these drugs through greater focus on reducing unnecessary or overzealous use. Instituting daily interruption of sedatives and analgesics, protocolizing their delivery, and instituting target-based sedation have all been shown to improve patient outcomes, although the studies reporting these results have not specifically looked at delirium rate or duration.107–109 Studies have also shown a benefit for pain control using morphine in the prevention of delirium, because pain itself can be a risk factor for the development of delirium. Family involvement can also be very helpful in reorienting and soothing some delirious patients. It is important to teach family members about the fluctuating course of delirium as well as how they can detect it. Preventive and management strategies for delirium in the ICU represent an important area for future investigation. Medications should be used for delirium only after adequate attention has been given to correction of modifiable contributing factors (e.g., sleep disturbance, restraints), as discussed previously. It is important to remember that delirium could be a manifestation of an acute, life-threatening problem that requires immediate attention (such as hypoxia, hypercarbia, hypoglycemia, metabolic derangement, or shock). After such concerns have been addressed, pharmacologic management should be considered. It should be recognized that although agents used to treat delirium are intended to improve cognition, they all have psychoactive effects that may further cloud the sensorium and promote a longer overall duration of cognitive impairment. Therefore, until we have outcomes data that confirm beneficial effects of treatment, these drugs should be used judiciously in the smallest possible dose and for the shortest time necessary, a practice infrequently adhered to in most ICUs. Indeed, some cases prove refractory to all “cocktail” approaches to sedation and delirium therapy, and in these cases, a trial of complete cessation of all psychoactive drugs should be considered. Some reports have described the utility of dexmedetomidine (an α2-agonist) as an adjunct to assist with weaning patients from all psychoactive medications.110 Preliminary results from a prospective, randomized, yet unblinded trial in postoperative patients who had undergone cardiac surgery showed that sedation with dexmedetomidine at sternal closure was associated with an 8% incidence of postoperative delirium compared with 50% for either propofol or benzodiazepines.111 Currently, no drugs are approved by the U.S. Food and Drug Administration (FDA) for the treatment of delirium. The SCCM guidelines89 recommend haloperidol as the drug of choice, with the acknowledgment that this recommendation is based on sparse outcome data from nonrandomized case series and anecdotal reports (i.e., level C data). Nevertheless, haloperidol is a “typical” butyrophenone antipsychotic, which is the most widely used neuroleptic agent for delirium.112 It does not suppress the respiratory drive and works as a dopamine receptor antagonist by blocking the δ2-opioid receptor, resulting in treatment of positive symptoms (hallucinations, unstructured thought patterns, etc.) and producing a variable sedative effect. There are data to suggest that haloperidol may have some anti-inflammatory properties,113,114 though this is theoretical in terms of a helpful pharmacologic effect on disease (such as septic delirium) at this point and requires further study. In the non-ICU setting, the recommended starting dose of haloperidol is 0.5 to 1.0 mg orally or parenterally, with repeated doses every 20 to 30 minutes until the desired effect is achieved. In the ICU, a recommended starting dose would be 2 to 5 mg every 6 to 12 hours (intravenous, intramuscular, or oral), with maximal effective doses usually in the neighborhood of 20 mg/day. This dose range will usually be adequate to achieve the “theoretically optimal” 60% δ2-receptor blockage115 while avoiding complete δ2-receptor saturation associated with the adverse effects cited later. Because of the urgency of the situation in many ICU patients—due to the potential for inadvertent removal of central lines, endotracheal tubes, or even aortic balloon pumps—much higher doses of haloperidol or a sedative are often used. Unfortunately, there are few data from formal pharmacologic investigations to guide dosage recommendations in the ICU. Once calm, the patient can usually be managed with much lower maintenance doses of haloperidol. Neither haloperidol nor similar agents (i.e., droperidol and chlorpromazine) have been extensively studied for ICU use.89 Newer “atypical” antipsychotic agents (e.g., risperidone, ziprasidone, quetiapine, and olanzapine) may also prove helpful for delirium.116
Delirium, Sleep, and Mental Health Disturbances in Critical Illness
Overview
Acute Brain Dysfunction or Delirium
Definition
Prevalence and Subtypes
Prognostic Significance
Pathophysiology
Neurotransmitters
Inflammatory Mediators
Impaired Oxidative Metabolism
Cholinergic Deficiency
Large Neutral Amino Acid in Delirium
Inflammation
Risk Factors for Delirium
Sedatives and Analgesic Agents Contributing to Delirium
Diagnosis
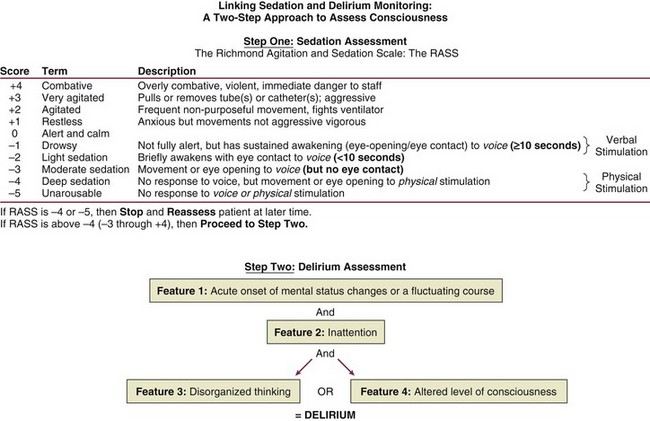
Prevention and Management
Primary Prevention and Nonpharmacologic Approaches
Pharmacologic Therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Delirium, Sleep, and Mental Health Disturbances in Critical Illness