Fig. 7.1
Polytrauma patient after damage control surgery: Pelvic clamp, external fixator, open abdomen with Vacu-seal
Apart from the basic treatment concepts of intensive care medicine (e.g., volume management, lung-protective respiratory support, nutrition, and anti-infectious therapy), treating physicians must be familiar with the trauma-induced cascades and trauma-associated MOD/MOF (e.g., coagulopathy, metabolism, thermoregulation). Insufficient cognition will promote mortality and morbidity caused by the alterations inherent to MOD/MOF.
7.2 Pathophysiology of Trauma
Severe trauma is characterized by a systemic reaction characterized by immunologic, neuroendocrine, microcirculatory, and coagulatory alterations. The functionally interwoven cascades are activated sequentially and in parallel. The typical findings are:
Acute phase reaction with the goal of activating the immune system, initiating a host defense and promoting reparative processes
Hyperinflammation (i.e., systemic inflammatory response syndrome [SIRS]) and increased endothelial permeability
Hypoinflammation progressing to immunoparalysis (i.e., compensatory anti-inflammatory response [CARS]) subsequent to the initial SIRS
Recruitment of leukocytes
Activation of the plasmatic coagulation cascades
Neuroendocrine response and metabolic alterations
Triggering, as well as modulation, of these trauma-related reactions results from disturbed microcirculation and alterations induced by ischemia/reperfusion.
To date, these trauma-induced cascades are best explained by a so-called two-hit model. While the first hit is induced by the initial trauma with soft-tissue damage, organ injury, and fractures, the second hit is triggered by the subsequent SIRS [1]. The second hit is caused by secondary insults resulting from endogenous or exogenous causes. Endogenous reasons are hypoxia, repetitive cardiovascular instability/hypovolemia, metabolic acidosis, ischemia/reperfusion, tissue necrosis, and infections associated with an antigenic load that activates the immune response (Fig. 7.2). Common exogenous reasons are extensive surgical interventions with additional tissue damage, extensive blood loss, disturbed coagulation, hypothermia, acidosis, mass transfusions, inadequate or delayed surgery, and inadequate or delayed intensive care treatment. Insufficient timely surgery and intensive care are reflected by the term “neglected trauma”.
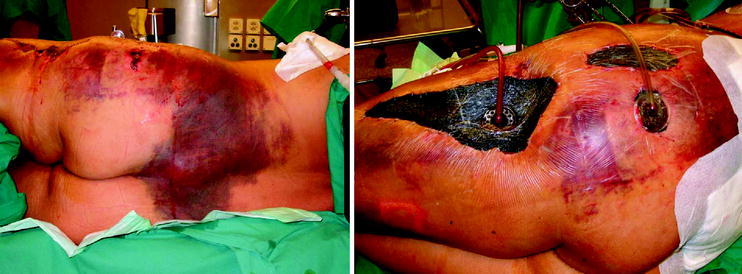

Fig. 7.2
Morel-Lavallee lesion of the pelvis (subcutaneous degloving) after débridement and vacuum sealing
These diverse alterations amplify inflammatory, neuroendocrine, and metabolic reactions [2].
The trauma and subsequent “antigenic load” induce local and systemic liberation of primary pro-inflammatory mediators followed by subsequent or parallel release of anti-inflammatory mediators.
The resulting extensive SIRS that can induce multiple organ dysfunction and progress to MOF substantially contributes to an increased morbidity and mortality. In this context, levels of cytokines as well as duration of elevated cytokine concentrations correlate with the severity of injury and are associated with an increased susceptibility to subsequent infections and mortality [3, 4]. In addition, the subsequent impaired cellular immunocompetence is clearly associated with an increased risk of developing sepsis that in turn is associated with an aggravated mortality following trauma [5, 6]. This explains why additional insults known to amplify destructive cascades must be avoided. Furthermore, perfect timing of potentially damaging interventions is indispensable.
Apart from the more obvious findings that can be measured and observed at the bedside, several additional factors have been identified. In this context, genetic predisposition and gender dependency influence morbidity and mortality:
Men show an increased morbidity and mortality compared with women [7]
Men show a significantly increased incidence of bacterial infections [8]
Women develop a sepsis significantly less often and thus have a better prognosis [9]
Male gender is a risk factor for developing pneumonia and septic complications following trauma [10]
Polymorphism of the interferon-γ-receptor-1-Gens is closely correlated with posttraumatic infections [11]
Aimed at reducing the “antigenic load” and thereby decreasing release of trigger factors, novel surgical strategic concepts during primary surgical care were developed and implemented in clinical routine. In this context, “damage control” has advanced to being an integral component in contemporary surgery [12]. A central element is to postpone the definitive surgical care of the severely injured patient and to initially perform less invasive primary surgical stabilization procedures (i.e., external fixation, tamponade for hemostasis). Definitive surgical care should be performed after the patient has been stabilized in the ICU. The different surgical steps are strongly influenced by the individual reactions with their individual temporal development (SIRS, CARS, infections, sepsis, and hemodynamic and cardiopulmonary instability) [13]. Consequently, intensive care is crucial in stabilizing and improving the condition of the patient, thereby preparing the patient for subsequent surgical interventions.
7.3 Intensive Care
In close cooperation with trauma surgeons, intensive care medicine entails the following duties and responsibilities:
Maintenance and restoration of vital and organ functions, including homeostasis concomitantly avoiding overcorrection
Optimization of overall condition for subsequent surgical treatment
Defining optimal time point for subsequent surgical treatment in cooperation with the trauma surgeons
Stabilization, prevention, and early diagnosis and treatment of general as well as trauma surgery-related complications
Successful treatment of severely injured patients in the ICU is comprised of different parts that can only be performed using an interdisciplinary approach. The following points are of particular importance:
Intensive nursing care/surveillance: Support of, and where necessary, taking over of activities of daily life (e.g., food intake, personal hygiene, movement, bedding) and medical attention and actions (e.g., analgesia, administration of fluids and drugs, complete control of vital functions and organ systems based on clinical surveillance, registration of monitored vital signs, handling of devices, etc.). Standardized physiotherapy aimed at improving and reinstituting breathing, mobilization, and movement.
Intensive care therapy: Consists of supporting the endogenous compensation mechanisms and reparative processes by optimizing substrate delivery (e.g., oxygenation, perfusion, nutrition), temporary artificial support of organ functions in the event of reversible organ failure, and prevention of secondary damage. The overall purpose is to create a condition allowing subsequent healing and recovery.
In this context it is of utmost importance to practise a holistic approach (i.e., to understand the patient) in its complete complexity and to guarantee an adequate and timely flow of information between the different disciplines involved. For the trauma patient, the interdisciplinary approach is indispensable to identify problems quickly, to react adequately, and to develop a strategy based on individual development and regression.
Many new procedures are considered impossible without contemporary intensive care medicine. A good example of this is the non-surgical management of patients with injuries to the liver, spleen, and kidney and is considered the best approach in hemodynamically stable patients. As recently reported, this non-surgical management of patients who are not actively bleeding is successful in >90 % of patents with isolated trauma and 94 % of multiply injured patients. This is only possible in the event of minimal volume administration, absence of brain injury, and additional abdominal trauma, as well as additional injuries requiring surgery and age below 55 years [14]. This, however, requires a continuous, competent, dedicated, and immediate readiness in the ICU.
7.4 Special Aspects in Trauma Intensive Care
Patients with traumatic brain injury (TBI), severe trauma, and multiple injuries are usually incapable of providing detailed information regarding the circumstances of the accident and their own personal medical history. Thus, additional injuries as well as concomitant pre-existing diseases and regular medication will remain unknown during the early posttraumatic phase and can be overlooked if information is not gathered from relatives and the treating general practitioner.
7.4.1 Hemorrhage
During the early posttraumatic phase, longstanding volume deficit and the hypovolemic-hemorrhagic shock are the most deleterious alterations that determine subsequent development and incidence of potentially devastating consequences. The most prominent pathophysiologic consequence is microcirculatory impairment, resulting from hypovolemia and subsequent sympathic-adrenergic precapillary vasoconstriction. These changes are accompanied by nitric oxide-induced vasodilation that in turn causes shunts and impairs nutritive capillary perfusion, explaining heterogenous capillary perfusion. As a consequence, impaired organ perfusion with evolving tissue acidosis and lactate production with sustained increase in endothelial permeability will aggravate the underlying condition as a result of progressive edema formation. In case of persisting ischemia, degradation of energetically rich phosphates in conjunction with free oxygen radical induced mitochondrial damage will result in irreversible structural and functional cell injury. The degree of the damage strongly depends on the extent and duration of the underlying hypovolemia. Reperfusion injury resulting from restored perfusion is feared for its generation of highly toxic free oxygen radicals. These, in turn, are known to damage cell membranes by peroxidation of cell membrane lipids, accounting for resulting vasoplegia and swelling.
The criteria defining diagnosis of hemorrhagic shock have been refined over the years. The early definition of hemorrhagic shock consisting of a lost blood volume of 1–2 l [15] was substituted by the systolic blood pressure <90 mmHg [16] and the vital parameters were used to estimate prognosis [17]. Based on the observation that the cardiovascular system is able to maintain an adequate systolic pressure by a compensatory increase in heart rate despite a progressive volume loss, the so-called shock index was introduced [18]. The shock index consists of an easy-to-calculate formula dividing systolic blood pressure by heart rate. A shock index <1 is highly suggestive of hemodynamic instability resulting from hypovolemia. In addition to the absolute values, duration of shock is also of crucial importance. In this context, a threshold of 70 min appears to be clinically relevant [19, 20]. Additional predictive factors are heart and breathing frequency upon admission to the hospital [21–23]. Especially in younger patients with well preserved compensation mechanisms macrocirculation (blood pressure, heart frequency, filling pressures) may appear “normal” although microcirculation still is impaired (i.e. serum lactate concentrations 12 hours after admission). This condition is referred to as “occult” hypoperfusion and is accompanied by an increase in infectious and other complications [24–25].
Diuresis can also be used as a parameter to estimate volume depletion, provided urine production and urine release are not hampered by pre-existing renal disease and injuries to the urinary tract system.
The overall accepted goal is to swiftly restore sufficient circulation, maintain hemodynamic stability by improving organ perfusion and microcirculation using volume administration via large bore catheters, and by reducing further temperature loss. The primary goal is quantitative restoration guided by hemodynamic parameters, restoration of peripheral perfusion, restitution of sufficient diuresis (0.5–1 ml/kg body weight, >2 ml/kg body weight in case of rhabdomyolysis, that is, creatin kinase (CK) >5,000 IU/ml), and reduction or normalization of arterial lactate, pH, and base excess values. In this context, lactate >2.5 mmol/l and negative base excess that has a higher negativity than – 8 mmol/l represent important threshold values of acidosis (pH < 7.2), and negative base excess values were shown to significantly predict outcome in traumatized patients [26].
Because low pH values did not unanimously correlate with the outcome, pH alone should not be used as a basis to limit therapeutic interventions. Predictive sensitivity is increased by the presence of other factors such as blood loss, hypothermia, increased lactate levels with negative base excess, and coagulopathy.
Perhaps more important than the absolute values is how long it takes to normalize lactacidosis and negative base excess during adequate treatment consisting of volume management, hemodynamic support, and rewarming. Persisting negative base excess and lactacidosis exceeding 24 h is clearly associated with significantly increased morbidity and mortality [24, 27]. Lactate-guided volume management was associated with significant reduction in mortality despite absence of improved signs of vasopressor-driven hemodynamic stabilization determined by measurements using the pulmonary artery catheter [24, 28].
7.4.2 Hypovolemia and Management of Hypovolemic/Hemorrhagic Shock
Qualitative volume replacement consists of substituting oxygen carriers (hemoglobin), factors of hemostasis (plasmatic coagulation factors, platelets), and correcting existing intravascular volume depletion. While a hemoglobin target of 9–10 g/dl had been targeted for many years, a lower hemoglobin count of approximately 7 g/dl was shown to improve outcome [29]. In this context, bedside point of care analysis of hemoglobin as well as glucose and lactate have significantly influenced morbidity and mortality and have reduced the number of resources consumed [30].
7.4.3 Disturbed Coagulation
Loss of coagulation factors and platelets as a result of uncontrolled hemorrhage, as well as dilution of coagulation factors and platelets resulting from excessive fluid replacement and reduced ionized calcium concentrations, hypothermia, and acidosis, in conjunction with the type and extent of injury (e.g., brain) all contribute to disturbed hemostatic mechanisms [31]. Contrary to the diffuse intravascular coagulopathy which includes thrombus formation we are confronted with traumatic intravascular coagulopathy (TIC) in which coagulation is hampered. In this context, the clinical picture of coagulopathy is not always reflected by laboratory values in a timely fashion. It is critical to base the subsequent administration of various coagulation factors on clinical judgment that, in turn, is strongly influenced by individual experience. Obvious bleeding requires immediate correction during the process of obtaining laboratory values that can take up to 60 min before results of plasmatic coagulation can be integrated into the fine-tuning of correcting TIC. Bedside analysis using thrombelastography may aid in faster and differentiated decision making. It is important to keep in mind that other elements apart from the concentration of coagulation factors and platelets are responsible for hemostatic failure [32]. An important devastating factor is underlying hypothermia that will disturb the entire coagulation cascade [33]. Inhibition of enzymatic reactions is reflected by prolonged prothrombin- and thromboplastin time during hypothermia even when the measured coagulation factors are normal. Another important technical detail is that functional coagulation tests are performed at 37 °C and not corrected for the actual temperature of the injured patients. This, in turn, will underestimate the extent of disturbed coagulation [34]. In addition, platelet function is impaired by hypothermia via reversible, temperature-dependent disturbance of thromboxane B2 production that will prolong the hemorrhage time [35]. Additional changes of the enzyme kinetics will delay initiation and propagation of platelet aggregation despite adequate platelet substitution [36]. This, in turn, explains the often seen poor correlation between platelet count and progressive bleeding in patients receiving massive transfusions and can be seen as an indication for platelet transfusion despite normal platelet count [37].
The following additional and preexisting coagulation disorders can aggravate the acute coagulation disorder: Release of tissue factors because of severe TBI, pharmacologic anticoagulation, functional disturbance of platelet functions due to pharmacological and endogenous (hepatic and renal insufficiency) influences, hemophilia and deficit in von Willebrand factor. Moreover, consumption of factors due to massive transfusions (MT) and underlying hemorrhage resulting in low 2,3-DPG concentrations, low activity of factors IV (ionized calcium), V, VIII, and XIII, low fibrinogen levels, dilution thrombocytopenia, functional platelet disturbance, hypothermia, and acidosis have to be taken into consideration. Massive transfusion is thought to increase citrate concentration which chelates calcium that in turn will impair the coagulation cascade and is also believed to increase protein C that can inhibit plasminogen activator inhibitor, thereby resulting in sustained activation of plasminogen; which can generate hyperfibrinolysis, and in turn, promote the development of microvascular hemorrhages at the mucosa, injuries, puncture sites, and sutures. Microvascular hemorrhages generally result from dilution coagulopathy and an increased consumption of hemostatic factors that can lead to diffuse bleeding that cannot be managed surgically. Consequently, the diagnosis of such a hemostatic disturbance must be made early to allow timely correction and prevention of aggravated complications. The diagnosis of such a hemostatic disturbance strongly depends on the vigilance and experience of the treating physicians in all involved disciplines.
Contrary to dilution coagulopathy, the combination of consumption and dilution coagulopathy will result in more severe disturbances seen in laboratory parameters reflected by a larger decrease in platelets, international normalized ratio, and fibrogen, as well as a pronounced prolongation of the activated prothrombine time (aPTT). Therefore, in patients requiring massive transfusion use of fresh frozen plasma (FFP) in order to provide a balanced set of activating and inhibitory coagulation factors (including Factor V) still may be indicated particularly because blood donors carrying a higher risk for induction of transfusion-related lung injury have been identified and excluded from FFP donation. This procedure is supported by a meta-analysis published in 2010 reporting that plasma infusion at high plasma: RBC ratios in patients undergoing MT was associated with a significant reduction in the risk of death [odds ratio (OR), 0.38; 95 % confidence interval (CI), 0.24–0.60] and multiorgan failure (OR, 0.40; 95 % CI, 0.26–0.60) [38]
Correction of disturbed coagulation occurs by supplementing the different components. To date there has been considerable controversy regarding correction strategies of impaired coagulation in trauma patients. Whereas in slight or moderate bleeding, targeted supplementation of coagulation factors monitored by means of thrombelastography or preferably thrombelastomety (i.e. ROTEM) as point of care testing (POCT) might be feasible, in severe and life-threatening bleeding this approach might fail because of the time lag between sample drawing and availability of the results. Therefore, as mentioned above use of fresh frozen plasma (FFP) in severe bleeding still is an important option in order to achieve hemostasis. However, certain factors, frequently fibrinogen and sometimes factor XIII must be additionally supplemented because provision by FFP alone might be insufficient. Factor XIII is a protein responsible for stabilizing the formation of a blood clot. In the absence of Factor XIII, a clot will still develop but it will remain unstable. If Factor XIII is deficient, the tenuously formed clot will eventually break down and cause recurrent bleeds. Suspicion in Factor XIII deficiency should be raised if in a patient with clinical relevant bleeding (i.e. diffuse micro vascular bleeding without clearly identifiable bleeding source if FXIII-activity is below 60 %). In such circumstances administration of FXIII in a dose of 30 IE/kg BW may be indicated. Furthermore, in trauma patients a positive influence on the development of the systemic inflammatory response syndrome (SIRS) could be demonstrated [39, 40, 41].
7.4.4 Hypercoagulability
Apart from possibilities of uncontrolled bleeding, trauma patients are exposed to a considerably elevated risk of thromboembolic complications in part because of an imbalance of pro- and anticoagulating factors and an increase in pro-coagulant factors (i.e., fibrinogen) resulting from a post-traumatic acute phase reaction.
Venous thromboembolic complications lead to a significant increase in morbidity and mortality and present in two forms:
Deep vein thrombosis
Lung embolism
A prophylaxis in trauma patients is important because clinical investigations for establishing a diagnosis are not sensitive enough. In a meta-analysis of 73 studies, however, it was found that none of the prophylaxis used was superior to another, even compared with no prophylaxis. Moreover, spinal injuries, spinal cord injuries, and age were identified as major risk factors for thromboembolic complications [42]. A prophylactic placement of caval filters may reduce the incidence of lung embolism [43, 44]. Our own experience demonstrates that cava filters can temporarily be inserted and removed in a high percentage of patients, and that they provide reliable protection against clinically relevant lung embolism [45–47].
7.4.5 Quantitative Volume Substitution
The choice of the type as well as of the amount of volume substitutes is still a matter of controversy. Young, healthy, trauma patients generally tolerate large amounts of intravenous fluids. In elderly patients, on the other hand, fluid and sodium overload may induce congestive heart failure, impaired blood–gas exchange, and hypoxia leading to the classic day 3 myocardial infarction and increased mortality, particularly if they are already suffering from a preexisting heart condition or renal insufficiency [48, 49].
It must be stressed that the classic 0.9 % sodium chloride solution is somehow toxic because it leads to hyperchloremic and dilution acidosis that exacerbates the existing acidosis in patients [48, 50, 51]. If the situation goes unrecognized, the attempt to correct the acidosis with additional fluid replacement will lead to a volume overload, with all its consequences [44, 52]. Additional unwanted effects of pure crystalloid fluid replacement are the decrease of cardiac output [53] and stroke volume by as much as 20 % even if adequate end diastolic pressure is achieved [44]. Furthermore, it has been shown that after pure crystalloid infusion – as opposed to higher concentration hydroxy ethyl starch – higher concentrations of pro-inflammatory cytokines, a depression of peritoneal macrophage function, and a higher expression of adhesion molecules can be found [54].
Patient’s microcirculation is very vulnerable to become compromised during the acute phase. Once a SIRS is induced, fluid overload and edema potentiate capillary leak leading to a local loss of control of inflammatory mediators and an increase of edema [55]. Furthermore, volume substitution with crystalloids alone leads to a reduced colloidosmotic pressure by up to 50 % with a consecutive fourfold increase of pulmonary transendothelial flux. Corrective administration of colloid fluids will consecutively reduce inflammation and edema [56–58].
In summary, volume expansion will improve cardiac output to a certain point via the Starling mechanism. Beyond that point it will, however, worsen cardiac function and promote edema formation, particularly if based solely on crystalloids [59]. Therefore, monitoring of stroke volume and cardiac output before and after fluid challenge is a more reliable alternative than assessing heart frequency and blood pressure alone [60–62].
7.4.5.1 Fluid Replacement and the Bowel System
In recent years, several studies have shown that an increased administration of sodium and water may have numerous detrimental effects on the gastrointestinal system: The edema in the splanchnic area leads to an increase in intraabdominal pressure that in turn can lead to a decrease of tissue oxygenation. Apart from intestinal permeability dysfunction, which is suspected to account for increased bacterial translocation, a protracted dysmotility of the bowel can be observed, causing intolerance to enteral nutrition. The resulting gastrointestinal dysfunction increases the risk of ventilation-associated pneumonia and therefore increases morbidity and mortality, as well as length of stay in the ICU and time to discharge [49, 59]. It is well recognized that the excessive use of crystalloids constitutes a risk factor for the development of abdominal compartment syndrome (ACS) in trauma patients. It has been shown that supranormal volume replacement, as it is often applied, will lead to an increase of the amount of crystalloid fluids infused, an increased incidence of ACS, and an elevated rate of MOF with a consecutively higher mortality rate [63–66]. Liberal infusion of crystalloid fluids in young trauma patients may lead to the secondary development of ACS even in the absence of abdominal injuries [67]. In most studies, the mortality rate resulting from ACS-induced lung failure or MOF is more than 50 % despite aggressive surgical abdominal decompression [63–66]. Administration of more than 3 l of crystalloid fluids prior to transfer of the patient from the Emergency Department to the ICU is highly predictive for the development of primary or secondary ACS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








