Gram-positive aerobic bacteria
Gram-negative aerobic bacteria
Anaerobic bacteria
Marine vibrio
Group A Streptococcus
Escherichia coli
Bacteroides sp.
Vibrio vulnificus
Group B Streptococcus
Pseudomonas aeruginosa sp.
Clostridium sp.
Vibrio parahaemolyticus
Peptostreptococcus sp.
Vibrio damsela
Staphylococcus aureus
Enterobacter cloacae
Peptococcus sp.
Vibrio alginolyticus
Coagulase-negative
Klebsiella sp.
Staphylococci
Proteus sp.
Enterococci
Serratia sp.
Bacilli
Acinetobacter
Citrobacter freundii
Pasteurella multocida
The cause of severe NSTI can be multi-bacterial.
The different bacteria may act in synergy.
In limb infections, only a single microorganism can be found, usually from the skin flora of the host, such as Staphylococcus pyogenes.
Under these circumstances the classification of NSTI is challenging. Ahrenholz developed a practical differentiation based on the classification by Lewis. The differentiation takes into account:
The level of the affected soft tissue, which determines the surgical intervention(s)
The responsible bacteria, which dictates the antibiotic therapy
9.4.1 NSTI of the Skin, Subcutaneous and Fascia Level
9.4.1.1 Cellulitis
Cellulitis caused by group A Streptococcus or Staphylococcus aureus is an acute infection of the skin and underlying soft tissues originating mostly from wound ulcer(s) or dermatosis. The classic sign of the infection is a hot, red, oedematous, sharply defined area. The patient may have fever, with lymphangitis and lymphadenitis possibly appearing later.
9.4.1.2 Synergistic Necrotizing Cellulitis
This type of infection is a variation of necrotizing fasciitis, which will be described later, and is caused by a combination of anaerobic bacteria and Enterobacteria. Synergistic necrotizing cellulitis is predominantly found in elderly patients with diabetes and cardiovascular and renal diseases, affecting mostly the lower extremities and the perineum, showing multiple cutaneous ulcers producing a reddish-brown fluid.
9.4.1.3 Clostridia Cellulitis
Clostridia is the cause of a specific type of cellulitis and typically appears 3–5 days after trauma. Characteristics are severe pain and the development of bullae that contain a reddish-brown fluid emitting a typical foul smell. In contrast to clostridia myositis, severe systemic septic-toxic reactions are absent.
9.4.1.4 Hemolytic Streptococcal Gangrene
Hemolytic streptococcal gangrene, described by Meleney in 1924, is characterized by a rapid spreading of infection and high fever developing within a short period of time. The local clinical signs are hyperemia, swelling, redness, and severe pain of the affected area. The skin is initially intact but within 2–4 days, the color changes from red to blue as a sign of ongoing subcutaneous spreading of infection with vascular thrombosis, ending in patchy skin gangrene. β-hemolytic Streptococcus is usually responsible for these infections and produces a number of toxins such as hemolysins, streptolysins O and S, and leukocidins, which cause local but severe systemic symptoms such as high fever, cardiovascular collapse, and respiratory problems.
9.4.1.5 Fournier’s Gangrene
In 1883, Fournier described a type of NSTI of the skin and soft tissue of the perineum and scrotum that progresses rapidly (<24 h) from the onset of the infection to the development of skin gangrene. The testicles are usually not affected. Patients complain about excruciating pain and show severe systemic toxic symptoms in the form of multiple organ dysfunction and eventually multiple organ failure frequently requiring intensive care treatment. Multiple organisms can cause this form of NSTI, but often the underlying cause is unknown. In 45 % of cases the infection originates from the urogenital system, in 33 % from the rectum, and in 21 % a local skin infection can be responsible for this form of infection.
9.4.1.6 Necrotizing Fasciitis
Necrotizing fasciitis is divided into types I and II. Type I necrotizing fasciitis is caused by mixed anaerobic bacteria (Enterobacteria and Streptococcus with Lancefield group A), whereas type II is caused by Streptococci with or without Staphylococcus. The synergism between these bacteria determines the progression of the infection. In contrast to β-hemolytic Streptococcus gangrene, this type of infection is not characterized by severe excruciating pain, and patients complain of only dull pain. In the early stage, systemic signs such as tachycardia, low blood pressure, and feeling unwell can be present. The spread of the infection within the subcutaneous level is always more extensive than it appears from the visible skin changes. On inspection, the tissue is grey or grey-green, while the muscle is initially not affected. The infection then rapidly progresses along the fascia (“highway to hell”) causing thrombosis of the subcutaneous blood vessels. At this stage the pain may decrease significantly as a result of the necrosis of the skin nerves. The nonexisting blood circulation within the skin causes necrosis, which is a late clinical sign in the infection.
9.4.2 NSTI of the Muscle
In this category NSTI are differentiated between
Myositis caused by Streptococcus
Myonecrosis caused by Clostridia
Often, in this type of NSTI, an entry point into the muscle layer cannot be identified. Most likely these infections are caused by hematological dissemination from a distant source within the body, in most cases an infected throat can be identified as the source.
9.4.2.1 Streptococcal Myositis
Streptococcal myositis is caused by Streptococcus of Group A and initially presents without myonecrosis. In some cases, a synergistic infection with Staphylococcus aureus has been described. The affected muscle tissue is discolored and swollen, but still functionally intact. At this early stage, the muscle tissue is not (yet) necrotic, however this type of infection is regarded as an NSTI because of the systemic reaction, which can deteriorate into a “Streptococcal toxic shock syndrome.”
9.4.2.2 Myonecrosis Caused by Streptococcus
Group A Streptococcus can cause myonecrosis, often in combination with Staphylococcus aureus and progress rapidly (<24 h). The affected muscle is unresponsive to stimulation and changes its normal color from red to grey. Because the vessels are thrombosed, no bleeding occurs in these necrotic areas unless the débridement reaches healthy muscle. The necrotic muscle areas are macerated, have lost their typical structural integrity, and can be easily débrided from the intact tissue. The margin of the infection is indicated by the appearance of bleeding, red color, and responsiveness of the muscle fibers to touch.
9.4.2.3 Clostridium Myonecrosis
Infections of muscle with Clostridium perfringes, novyi, and septicum can be found after severe agricultural accidents, perforating injuries, or in patients with intestinal tumors (Fig. 9.1). Clostridia are part of our normal environment and intestinal flora. The clinical features are severe pain and profound systemic reactions in the form of septicemia with high fever, coagulopathy, and initially, multiple organ dysfunction, often developing into a multiple organ failure. The main characteristic is a sweet-foul smell from the wound secretion. Clostridia produce α-toxin (lecithinase), which causes cell membrane rupture leading to hemolysis and deactivation of white blood cells. It has also a cardiotoxic effect. This form of NSTI progresses quickly. Within hours, the skin, which is initially edematous, changes from pale to blue with the appearance of hemorrhagic bullae filled with non-purulent fluid. The texture of the affected muscle is edematous and pale in color, before it becomes gangrenous and black. Characteristically, the affected muscle is unresponsive to stimulation and extremely friable. Patients with diabetes or angiopathy are more susceptible to this type of NSTI. Increase in serum creatinine kinase and urine myoglobin values indicate muscle involvement in this type of infection. Gram staining of the fluid from the affected area shows the characteristic form of Clostridia (i.e., boxcars). Positive blood cultures for Clostridia may be found in only 15 % of patients.
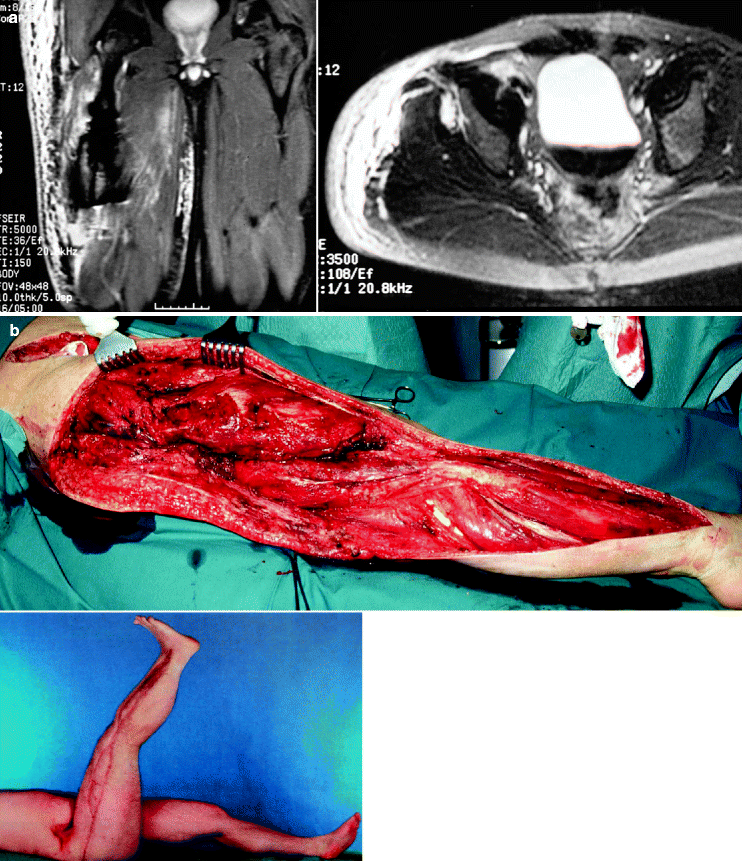

Fig. 9.1
(a) Myonecrosis of right thigh because of Clostridium perfingens: MRI at admission. (b) Status after radical débridement including right retroperitoneal space, several “second-look” procedures, and the final result after wound healing and skin grafting
9.4.3 Toxic Shock Syndrome
Willoughby and Greenberg introduced the definition of “Streptococcal toxic shock syndrome” in 1983, similar to the definition of the Staphylococcal toxic shock syndrome. Several consensus criteria must be satisfied for the diagnosis of this syndrome, such as isolation of Streptococcus strains from the patient and certain clinical signs such as erythema, muscle necrosis, hypotension, renal insufficiency, coagulopathy, liver dysfunction/failure, and adult respiratory distress syndrome.
Toxic shock syndrome (TSS) develops in 50 % of the patients who suffer from necrotizing fasciitis as a result of Streptococci infections, and 60 % of the infections originate from the skin or urogenital tract. In other instances, a translocation of bacteria from the pharynx into the bloodstream is responsible for the infections.
Three clinical phases of TSS:
In the first phase, the symptoms are unspecific and present as generalized or localized muscle pain, vomiting, general malaise, and diarrhea. The shock symptoms can develop hyper-acutely within hours, but can also take several days, depending on the causative bacteria, the localization of the infection, and the immune status of the patient.
In the second phase, the pain and swelling increase in the affected side. Patients show systemic toxic signs such as tachycardia, tachypnea, and high fever. Rarely is lymphangitis or lymphadenitis seen.
Without adequate therapy the infection progresses to the third phase, which is a severe TSS with multiple organ failure. An important fact is that TSS has been seen in patients with and without skin gangrene.
The shock symptoms are caused in part by so-called “superantigens”, which are proteins produced by certain group A Streptococci, but also by Staphylococci (exotoxine type A, B, C, D, F, MF, SSA). The difference of these proteins as compared with conventional antigens is that they are not controlled by the major histocompatibility complex of T cell receptors. The consequence is that normal T cell activation is bypassed and massive and uncontrolled amounts of immune mediators (e.g., tumor necrosis factor-α, interleukin-1, -2, -6, -8, -12, and interferon-γ) are liberated into the circulation of the affected patient, causing the described shock symptoms.
9.4.4 Diagnosis
9.4.4.1 Clinical Signs
Early diagnosis of NSTI is often difficult because of the lack of typical clinical signs and the absence of characteristic skin changes. As a general rule, skin necrosis is a late sign of NSTI. However, the survival of patients with NSTI depends on early diagnosis, which can only be made by a physician who approaches the patient with a high suspicion for NSTI, if the following clinical signs are present:
Symptoms of severe local or systemic infection
Systematic toxicity
Radiograph showing subcutaneous gas
Histology with characteristic changes for ongoing severe infections
Positive microbiology
One feature in particular that must always alert a clinician for considering NSTI is pain that is disproportionate to the clinical findings. Sudden decrease in pain or anesthesia at an apparent site of infection is a late change and is a result of necrosis of the skin nerves.
In patients in whom NSTI is suspected, all body surfaces must be inspected during the physical examination.
The skin must be examined for:
Erythema
Tense skin edema
Grayish or other discolored wound drainage
Vesicles or bullae
Necrosis
Ulcers
Crepitus
Bullae with serous fluid appear as an early clinical sign during the course of the infection, whereas large hemorrhagic bullae, skin necrosis, crepitus, and sensory and motor deficits are late signs. Migration of the margins of erythema and increasing skin induration despite the use of intravenous antibiotics is another indication of NSTI.
Clinical symptoms such as hypotension, fever (temperature > 38 °C), tachycardia, tachypnea, mental disturbance, and tremor may or may not be present or appear suddenly without any warning. Marked increase in white blood cell count and metabolic acidosis often occur at a later stage and reflect severe sepsis.
9.4.4.2 Radiology
NSTIs can cause subcutaneous gas produced by bacteria that can be detected using various radiography techniques. Gas in soft tissue may be visible on conventional radiographs, ultrasonography and computed tomography (CT). Compared with plain-film radiography or ultrasonography, CT and magnetic resonance imaging (MRI) are superior for detecting tissue inflammation and necrosis. Gadolinium-enhanced MRI demonstrates not only gas within the soft tissue, but marks the extent and the involvement of the different tissue layers. It is important to understand that these examinations show the extent of the infection at the time of the examination, but the infection continues to progress at a high speed. The area of NSTI may continue to increase until the patient has been transported to the operating room for surgical treatment.
9.4.4.3 Laboratory Tests
Patients with NSTI have
Abnormal blood values
Elevated polymorphonuclear leucocyte counts
Anemia
Hypocalcaemia
Acidosis
Decreased platelet counts
Increased prothrombin and partial thromboplastin time
Elevated serum creatinine phosphokinase
The combination of local symptoms, systemic toxicity, radiological results, and pathological laboratory tests should raise the suspicion of NSTI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








