Fig. 19.1
A case of fibrous dysplasia of the proximal femur causing a pathologic fracture
Chronic Osteitis: There are usually obvious bony changes with cavitation, sequestrum, and involucrum formation [5].
Non Ossifying Fibroma: Presents between the ages of 10 and 20 years. They are metaphyseal lesions mainly in the femur or tibia. They present as osteolytic eccentric lesions, generally oval in shape. Multilocular appearance or ridges in the bony wall may be seen (Fig. 19.2) [9].
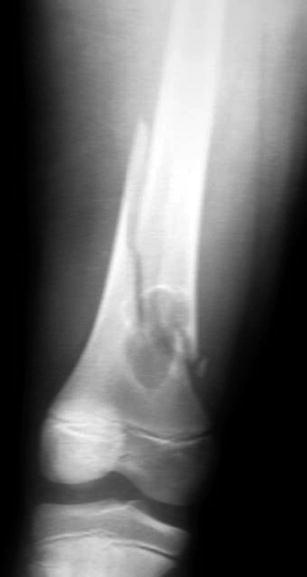

Fig. 19.2
Non-ossifying fibroma of the distal femur with a pathologic fracture
Unicameral Bone Cyst: Usually presents between the ages of 5 and 15 years. It is usually seen in the proximal metaphysis of the humerus or the femur. It presents as a central osteolytic lesion extended to the whole cross section of the bone. It may slightly expand the cortex. The cortex remains well defined and with no periosteal reaction. Pathologic fractures through the cyst may show the “falling leaf” sign (Fig. 19.3) [6].
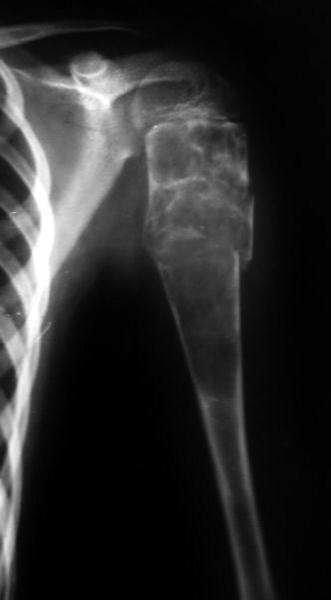

Fig. 19.3
Simple bone cyst with secondary aneurysmal changes and a pathologic fracture of proximal humerus
Aneurysmal Bone Cyst: Most cases are seen before the age of 20 years. The lesion is purely osteolytic, metaphyseal in long bones, and usually eccentric. The cortex is attenuated or completely destroyed. The periosteum is often elevated in a blow-out image with no apparent demarcation with the soft tissues [6].
Enchondroma: They are found mostly in the tubular bones of the hand, but can be seen in other long bones such as the femur. They are seen as osteolytic, round or lobulated lesions, with well-defined edges. They are usually central and metaphyseal. They sometimes contain opacities producing a “popcorn” appearance [5, 9].
Chondoblastoma: It is usually epiphyseal, occurring in adolescents. The proximal humerus is the most common site. It shows as an osteolytic lesion centered in the epiphysis with the physeal plate open or just closed. The osteolysis is usually rounded and well-defined by a thin rim of osteosclerosis. It may be spotted by granules of fading radiodensity. The cortex may be expanded, and the metaphysis invaded [9].
Giant Cell Tumor: Found usually at the ends of long bones and 50 % are found around the knee. They involve both the epiphysis and the metaphysis. The lesion is intramedullary and purely osteolytic. The cortex is generally moderately expanded and constituted by a thin shell of reactive bone [5, 9].
Ewing’s Sarcoma: Appears roentgenographically as a destructive lesion in the diaphysis of long bones. Patients are typically under the age of 15 years. The lesions show characteristic “onion skin” appearance. It is not uncommon for a large portion of the bone to be involved [13].
Osteosarcoma: Classic osteosarcoma is metaphyseal, usually around the knee or in the proximal humerus. The lesion is intramedullary with a combination of radiolucency and osseous radiodensity. It may breach the cortex and expand towards the soft tissues. The soft-tissue extension may show stripes of radiodensity perpendicular to the cortex “sun-ray appearance” [5, 9, 11].
Multiple Myeloma: Appears roentgenographically as multiple “punched-out”, sharply demarcated, purely lytic lesions without any surrounding reactive sclerosis. There is usually obvious osteopenia [5, 9].
Lymphoma: Typically appears as an ill-defined area of bone destruction, usually diaphyseal, and often with a permeative appearance. Periosteal reaction is rarely seen and frequently a large portion of the bone may be involved [5, 9].
Bone Metastases: The roentgenographic appearance of metastatic carcinoma is variable. However, the appearance is usually aggressive, suggestive of malignancy. It should be remembered that in any patient over the age of 40 years, even without a history of malignancy, an aggressive-appearing bone lesion is most likely metastatic or a result of multiple myeloma. The lesions can be lytic, blastic, or mixed. Most metastases are purely or predominantly lytic, as are those from the kidney, lung, breast, thyroid, gastrointestinal tract, and melanoma. Carcinomas of the kidney or thyroid are usually associated with pure osteolysis and a blow-out appearance of the bone. Blastic metastases are those mainly from prostate cancer. Mixed blastic and lytic metastases are particularly frequent in breast and lung carcinoma, but can also be seen in carcinomas of the gastrointestinal tract. Metastases distal to the knee or elbow are rare, and in such cases, lung cancer is the most likely cause. The most common locations for bony metastases are the spine, ribs, pelvis, femur, and humerus [9, 14].
Localization also depends on the primary carcinoma. Breast and thyroid metastases prefer the locations of the trunk, proximal humerus, proximal femur, and skull. Prostate, rectum, uterus metastases frequently occur in the lumbar spine, sacrum, pelvis, and proximal femur. The intracortical or subperiosteal metastases of the shafts are typical of lung carcinoma, as are the rare metastases to the hand, while the metastases to the foot are usually because of carcinomas of subdiaphragmatic organs.
Metastases from the breast and prostate tend to become widely disseminated, while those from the kidney and thyroid may remain solitary for an extended period of time [14, 16, 18].
CT and magnetic resonance imaging (MRI) of the affected bone: CT is superior in delineating osseous details and destruction. MRI is particularly important for showing marrow changes and extent of soft-tissue spread. It is highly important in the staging of some primary malignant bone tumors. The amount of soft-tissue extension and the number of muscle compartments invaded as shown by MRI can have great relevance as to the decision of management of primary malignant bone lesions. It is to be emphasized that all local radiologic examinations must be fulfilled before a biopsy is performed as this will distort the local anatomy, and usually gives an impression that the tumor is larger than it actually is [15, 19].
Angiography: May be needed as part of the preoperative planning in some fractures. It helps to assess the proximity, and possible invasion, of the vascular bundle which holds importance in the decision of limb salvage versus amputation regarding some malignant bone tumors, and in properly planning the surgery. Preoperative embolization may also be needed in some highly vascular lesions (e.g., metastases from kidney and thyroid) [2, 9, 14].
PET: Positron emission tomography (PET) images glucose metabolism, and therefore gives a non-invasive reliable method of assessing the grade of malignancy of bone tumors. It is to be noted, however, that although metabolic activity is usually correlated with the degree of malignancy, some benign tumors may show marked metabolic activity (e.g., giant cell tumor). Post-treatment reduction in glucose metabolism by the tumor is useful in assessing the response to chemotherapy, and radiotherapy. PET is also a useful tool in the detection of bone metastases [5, 9, 14].
Bone scan: Is highly sensitive in picking up multiple bone lesions (e.g., metastases or skip lesions). Bone scans should be obtained in all cases with bone malignancy. Hot areas seen on the scan are then correlated with plain radiography. It should be remembered that some lesions (e.g., myeloma) present as cold areas [5, 9].
Chest radiograph: Are obtained in all cases as a part of preoperative assessment, to rule out primary lung tumors, or lung metastases.
CT chest is important before the definitive management of some primary malignant bone tumors known to metastasize early to the lung (e.g., osteosarcoma). It may also show a primary lung carcinoma in metastatic disease.
CT abdomen and pelvis are important in locating the primary source of malignancy, and in the staging of some tumors (e.g., lymphoma).
Other tests used in evaluating a patient with a suspected pathologic fracture of unknown etiology include:
Mammography.
Upper and lower gastrointestinal series.
Endoscopy.
Liver, spleen, and thyroid scans.
Intravenous pyelogram and renal ultrasound.
It should be noted that despite fulfilling all the mentioned examinations, the primary source will not be found in approximately 15 % of patients with suspected metastatic bone disease [5, 9].
19.10 Management and Preoperative Planning of Pathologic Fractures
Before proceeding with management of any patient with pathologic fracture, careful preoperative planning must be undertaken to evaluate the case, confirm the diagnosis, and assess the general condition. This includes clinical evaluation, laboratory investigations, radiologic examination, and finally biopsy in certain cases. The goal is to confirm the diagnosis, assess the severity and extent of the bone lesion, document the stage in primary malignant conditions, and assess the general condition of the patient. It is to be emphasized that a team approach to the preoperative planning in the management of pathologic fractures caused by malignancy should include not only the surgeon, but also the oncologist, pathologist, radiologist, internal medicine physician, and radiation therapist [2, 3, 5].
19.10.1 Bone Biopsy
Before proceeding to the management of a bone lesion with impending fracture, or with a pathologic fracture, every effort must be made to clarify the nature of the lesion including clinical, laboratory, radiologic investigations, and finally, the use of bone biopsy. This is not required in patients with known malignancy and multiple metatstases.
General Biopsy rules:
A biopsy should be planned as carefully as the definitive procedure. It should only be done after all the clinical, laboratory, and roentgenographic examinations are fulfilled. It is preferably done in the same center where the definitive surgery will take place. It has its unique role in diagnosis of primary bone lesions, and other bone lesions of unknown aetiology. Solitary metastasis in a patient with known malignancy should also be biopsied.
A biopsy can be done using fine-needle aspiration, core needle biopsy, or an open incisional procedure. Incisional biopsy is associated with complications, but is least likely to be associated with a sampling error and provides enough tissue for additional diagnostic studies. Core needle biopsy is the preferred technique at our facility. It should be remembered that whether a needle or incisional biopsy is done, the biopsy track should be considered contaminated with tumor cells [5, 20].
The general rules for biopsy include:
The biopsy track should be in line with the incision of the definitive surgery as it must be completely excised in the definitive surgery.
We do not favor the use of tourniquets, however, if a tourniquet is used, then the limb may be elevated but should not be exsanguinated by compression.
Transverse incisions should be avoided, and in an extremity, the incision should be as distal as possible.
The incision should move directly toward the tumor passing through a single muscle rather than in an intermuscular plane.
If the tumor has a soft-tissue component, then this is best biopsied.
If a hole must be drilled in the bone (not in cases presenting with a pathologic fracture) then it must be oval or round and not act as a stress riser and should be sealed by the least amount of bone cement to not push the tumor up or down.
The periphery of the lesion is the most viable tissue to be biopsied as the center usually yields necrotic tissue.
Complete hemostasis should be achieved by the end of the procedure.
The definitive procedure can be carried out immediately depending on the results of a frozen section only if it confirms the clinical and radiological diagnosis. Otherwise, the definitive procedure should be delayed until a definite diagnosis is reached.
Regarding small lesions, or lesions in difficult sites where the accuracy of the conventional intraoperative C-arm can be limited, we have found a great value for the use of CT-based 3D navigation system or 3D C-arm imaging to accurately localize those lesions intraoperatively [22].
19.11 Treatment of Pathologic Fractures
Treatment of pathologic fractures is not necessarily surgical and depends on the definite pathology and on the fracture site and geometry among other parameters. It is to be noted that bones affected by a local pathology do have the ability to heal after a fracture although the healing time is usually slower than normal bone, particularly after radiation therapy or chemotherapy.
Initially, all patients with pathologic fractures should be offered the usual fracture care with immobilization and reduction. Optimization of the medical condition in elderly or fragile patients should start. Evaluation of the underlying pathologic process is a must with a full clinical work up (as formerly stated), laboratory investigations, radiology, and finally biopsy for unknown or locally destructive lesions [5].
19.11.1 Non-surgical Treatment
Fractures through certain benign bone lesions that can be reduced and maintained conservatively can be managed non-surgically. Examples include certain fractures associated with rickets, unicameral bone cyst, osteoporosis, or osteopetrosis [5, 6].
Radiotherapy is considered the mainstay of treatment of metastatic disease to the spine from a radiosensitive tumor unless there is gross instability or if neurologic deficit occurs [23].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








