FIGURE 25-1. The Codman 3000 nonprogrammable, implantable intrathecal drug delivery pump. With the Codman system, intermittent injections can be given through a closed tip needle with a needle shaft aperture that directs fluid away from the drug reservoir chamber and directly into the catheter. Pump refills utilize an open tip needle without a needle shaft aperture, directing fluid into the inner drug reservoir chamber. Thus, the Codman system has required reliance upon proper needle type for refill or bolus in order to minimize the risk of overdose. (Image courtesy of DePuy Spine Inc., Raynham, MA.)
The Shiley-Infusaid pump had two potential sources of access, an inlet septum for access to the inner drug reservoir chamber during refill and a side port for intermittent injection. Unfortunately, there was no inherent fail-safe system to distinguish between the two ports, with potentially dire consequences. With the present Codman system (Fig. 25-1), intermittent injections can be given through a closed tip needle with a needle shaft aperture that directs fluid away from the drug reservoir chamber and directly into the catheter. Pump refills utilize an open tip needle without a needle shaft aperture, directing fluid into the inner drug reservoir chamber. Thus, the Codman system has required reliance upon proper needle type for refill or bolus in order to minimize the risk of overdose.
While Medtronic Inc. (Minneapolis, MN) manufactures a constant flow pump (Isomed System), it is best known for its production of programmable intrathecal drug delivery systems, specifically the Synchromed, Synchromed-EL, and now the Synchromed 2 (Fig. 25-2). The original Synchromed device entered the marketplace in 1991. The design utilizes the technology of pacemakers to allow programmability. The pump consists of a lithium battery, a reservoir system, microprocessor, and antenna. An external programmer can communicate with the pump to change settings, allowing programmability.

FIGURE 25-2. The Medtronic Synchromed II programmable, implantable intrathecal drug delivery pump. The design utilizes the technology of pacemakers to allow programmability. The pump consists of a lithium battery, a reservoir system, microprocessor, and antenna. An external programmer can communicate with the pump to change settings, allowing programmability. Access to the side port is limited by a wire mesh over the entry port that restricts needle access to 25-gauge or smaller needle. The side port connects directly to the intrathecal catheter, and access can allow sampling of CSF as well as direct administration of drug boluses. Limiting needle access to the sideport to smaller gauge needles reduces the chances of inadvertent administration of the reservoir refill directly in to the intrathecal space. (Image courtesy of Medtronic Inc., Minneapolis, MN.)
Implanted catheters with subcutaneous injection sites have been developed as intrathecal drug delivery systems such as the Port-a-Cath system (Fig. 25-3, Smiths Medical, St. Paul, MN). Such a design is not useful for long-term delivery (years) given it is implicitly an “open” system with the need for repetitive site instrumentation, potentially leading to infectious complications. Implanted catheters with subcutaneous injections sites do have utility for epidural administration of short to intermediate duration. The remainder of this chapter is limited to a discussion of complications associated with implanted intrathecal drug delivery pumps,which are now in common use for the treatment of a wide range of pain disorders (Box 25-1).
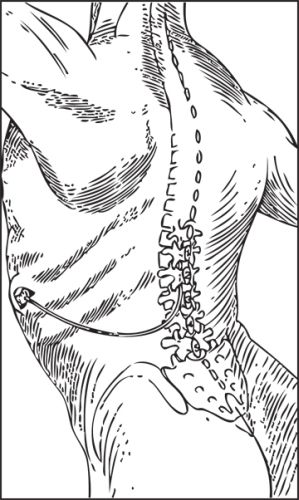
FIGURE 25-3. The Port-a-Cath implanted catheter with subcutaneous injections port. These percutaneously accessed systems have been used to provide long-term intrathecal infusions using an external reservoir and drug delivery pump. Such a design is not useful for long-term delivery (years) given it is implicitly an “open” system with the need for repetitive site instrumentation, invariably leading to infectious complications. Implanted catheters with subcutaneous injections sites do have utility for epidural administration of short to intermediate duration. (Illustration redrawn to illustrate the Port-a-Cath system, Smiths Medical, St. Paul, MN.)
BOX 25-1 Established Indications for Intrathecal Drug Delivery
Controlled trials support the use of intrathecal drug delivery for the following indications:
 Spasticity associated with cerebral palsy and spinal cord injury unresponsive to oral agents
Spasticity associated with cerebral palsy and spinal cord injury unresponsive to oral agents
 Chronic cancer-related pain poorly responsive to more conservative treatment
Chronic cancer-related pain poorly responsive to more conservative treatment
Limited observational data support the use of intrathecal drug delivery for the following indications:
 Chronic low back pain
Chronic low back pain
 Complex regional pain syndrome (CRPS)
Complex regional pain syndrome (CRPS)
 Other forms of chronic neuropathic pain
Other forms of chronic neuropathic pain
 SCOPE
SCOPE
Complications associated with intrathecal drug delivery systems can be categorized as surgical, infectious, device related (catheter and/or pump), and drug related. Intrathecal drug delivery systems have been in widespread use for more than two decades, and as a result there is significant experience with the complications associated with use of these devices. The reported incidence of adverse events ranges from 3% to 24%, most of which are minor and related to the infused drug.9 Most device-related complications occur at the time of or within the first few months after implantation, and many of these surgical complications can be avoided with careful surgical technique and recent improvements in technology. In the largest available series, complications related to the device or catheter occurred in 21.1% of patients during the first 9 months after implantation.10 Drug-related complications are common and typically evolve over several months following implantation.9 In recent years, it has been recognized that long-term intrathecal drug delivery can lead to formation of inflammatory masses at the tip of the intrathecal catheter, within the thecal sac. Since the first reports of inflammatory mass formation, there has been increased reporting of this complication, and these reports have led to greater concern about the risk of significant neurologic injury.11 Recent postmarketing surveillance reports alerted the manufacturer of the Synchromed device to a series of deaths that occurred early after pump implantation; this led to a large-scale epidemiologic study, which concluded that intrathecal drug therapy is associated with a significant increase in mortality rate.12 The reasons for this increase are unclear, but possible mechanisms will be discussed below.
 DEFINITION, INCIDENCE, AND DIAGNOSIS
DEFINITION, INCIDENCE, AND DIAGNOSIS
Surgical Complications
Intrathecal drug delivery involves the placement of an indwelling catheter within the thecal sac, and injury to the neuraxis can occur in several ways. During initial implantation, direct trauma to the spinal cord or spinal nerves can result from needle or catheter placement. Because the spinal nerves of the cauda equina float freely within the cerebrospinal fluid (CSF) below the L1-L2 level, this problem is unlikely if needle entry is below this level. Catheter complications can result from threading the catheter into the conus medullaris or other portions of the spinal cord, resulting in catastrophic neurologic injury, typically with paraparesis or complete paraplegia ensuing.13,14 During direct placement of the catheter into the spinal cord parenchyma at the time of implantation, the awake patient is likely to report pain or paraesthesia, particularly reports of pain involving both sides of the trunk or both lower extremities. This complication may be more likely when the patient undergoes implantation while under general anesthesia, and some authors have concluded that pumps should be implanted under sedation only whenever possible, with a level of sedation that allows for verbal communication with the patient throughout catheter placement.13 Other complications that may occur at or soon after the initial surgical period include CSF leak, hygroma formation, and chronic postdural puncture headache. The incidence of common complications occurring within the first months after implantation is shown in Table 25-1.
TABLE 25.1 Frequency of Complications Associated with Implantation of Intrathecal Drug Delivery Systems
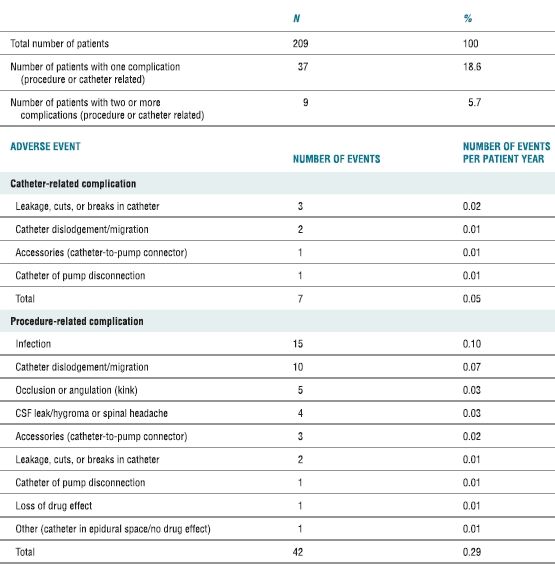
Reproduced from Follett KA, Naumann CP. A prospective study of catheter-related complications of intrathecal drug delivery systems. J Pain Symptom Manage 2000;19:209–215, with permission.
The tissue dissection required to place an intrathecal drug delivery system is minimal and limited to the subcutaneous tissues. Nonetheless, significant bleeding can occur and lead to the need for wound reexploration and evacuation of hematoma in the immediate postoperative period. Bleeding within the pocket can lead to the formation of a significant hematoma and, if left untreated, may lead to wound dehiscence. The diagnosis is typically obvious: the patient reports pain immediately postoperatively that is accompanied by progressive swelling at the surgical site. Bleeding within the spinal canal can also occur after intrathecal catheter placement, resulting in epidural hematoma formation. Close attention should be paid to evaluation of the patient who presents with worsening back pain in the immediate postoperative period; the appearance of neurologic deficits warrants emergent imaging (magnetic resonance imaging [MRI] or computed tomography [CT]) and evacuation of the epidural hematoma, if present.
Leakage of CSF can result in immediate postprocedural headache and has the typical characteristics of a post dural-puncture headache: lack of any alternate cause and the onset of worsening of headache related to sitting upright or standing. While this headache is typically self-limited and responds to conservative treatment, the CSF leak can be persistent and lead to chronic positional headache. Ongoing CSF leakage can also flow along the course of the tunneled catheter from the thecal sac all the way to the subcutaneous pocket in the paraspinous region where the catheter is typically fastened to the paraspinous fascia. These subcutaneous CSF collections (termed a “hygroma”; Fig. 25-4) can become quite large and may even lead to breakdown of the overlying incision. The diagnosis of a CSF hygroma is suspected in the patient who presents with a painless, fluctuant swelling in the area underlying the paraspinous incision made at the time of catheter placement and is best confirmed using MRI.
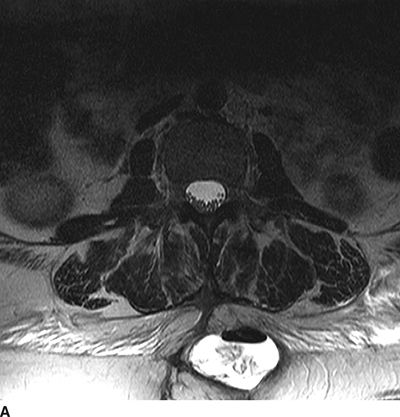
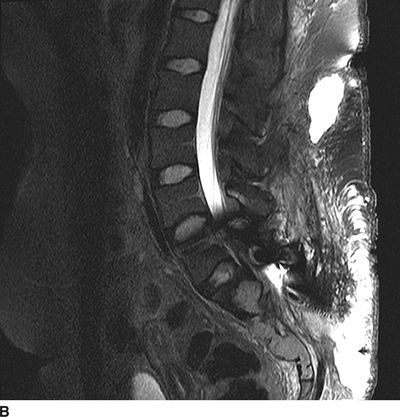
FIGURE 25-4. Subcutaneous CSF hygroma associated with an implanted intrathecal drug delivery system as seen on MRI. A: Axial T2-weighted MRI of the lumbosacral spine. B: Sagittal T2-weighted MRI of the lumbosacral spine. This patient presented with a large, fluctuant area just beneath the lumbar paraspinous incision. There was no pain, tenderness or erythema in the region. MRI demonstrates a large, high-signal fluid collection with an air-fluid level within located in the right paraspinous subcutaneous region where the intrathecal catheter was anchored to the subcutaneous tissue during implantation. Surgical exploration revealed clear fluid without evidence for infection. The catheter was removed and the pump was left in situ; several months later the catheter was replaced and connected with the existing pump and intrathecal therapy was resumed without incident.
Catheter kinks, breaks, leakage, dislodgement from the CSF, and disconnection from the pump all occur with some regularity. Catheter-related complications occurred immediately at the time of implant in 3.3% of cases in one large series; catheter dislodgement also occurred weeks or months after device placement in 9.7% of cases. The diagnosis of catheter-related complications can be quite difficult. The most common feature is unexplained, inadequate pain control. This leads to a search for the cause, and this search usually ensues after several dose escalations fail to provide pain relief. The diagnosis is best made by careful examination of the entire length of the catheter from the point where it attaches to the subcutaneous pump all the way along the subcutaneous course to the intrathecal tip of the catheter. When the catheter is completely dislodged from the intrathecal space, disconnected, or severed, this is obvious on simple radiographic inspection using fluoroscopy, x-ray, or CT (Fig. 25-5). Catheter kinks or leaks caused by small holes or breaks in the catheter are more difficult to diagnose. The best means is to access the intrathecal pump through the side port (Fig. 25-6), aspirate enough CSF to clear the catheter of drug, and inject nonionic radiographic contrast medium that is safe for myelography. Leaks along the course can be seen as the radiographic contrast leaks in to the subcutaneous tissue. Correct intrathecal placement will be apparent when a normal myelogram appears; subtle misplacement, for example, subdural or epidural catheter location, can also be readily detected in this manner.
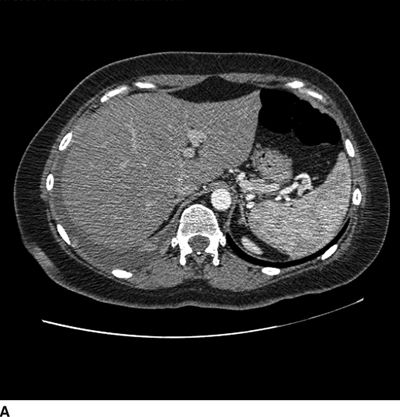
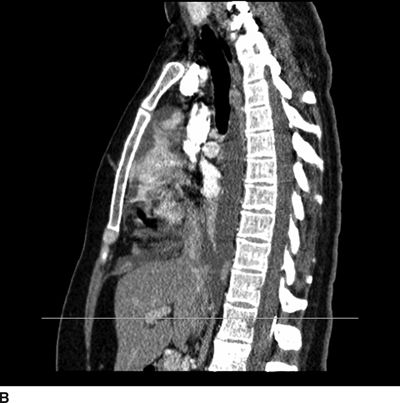
FIGURE 25-5. Sagittal (A) and axial (B) CT of the thorax in a patient with an intrathecal drug delivery system placed and providing pain relief for a patient with chest wall pain associated with metastatic lung cancer. The catheter tip can be seen in the midline in the posterior aspect of the thecal sac at the T9/T10 level. Reference line on the sagittal images corresponds with the level of the axial image shown.
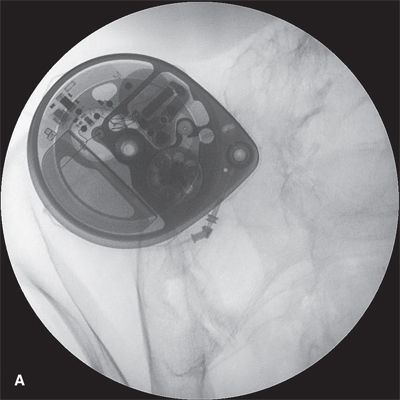
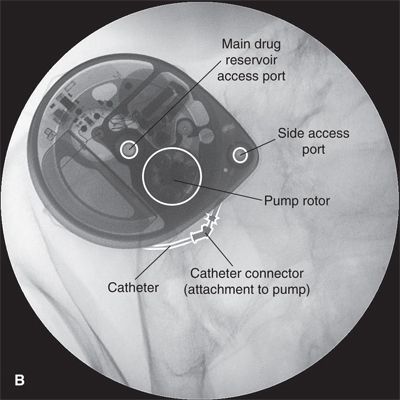
FIGURE 25-6. A: Appearance of the Medtronic Synchromed II (Minneapolis, MN) 40 mL intrathecal drug delivery pump as seen in situ using fluoroscopy in the anterior-posterior plane. B: Labeled image. Fluoroscopy can be used to readily identify the drug reservoir access port during routine periodic refilling of the pump using the 22-gauge Huber-type (non-coring) needle supplied by the manufacturer. By taking two sequential radiographs separated by several minutes, fluoroscopy can also be used to assess proper rotation of the rollers around the rotor in the peristaltic pump, as their position will change if the rotor is moving. Finally, fluoroscopy is essential when assessing the integrity of the catheter and its position within the CSF using the side access port. The side access port can be accessed with a 25-gauge needle; the side access port is specifically designed to prevent entry with the larger needle used for drug refills. Once the needle is in position, at least 0.3 mL of fluid must be withdrawn to clear the catheter of highly concentrated drug and prevent administration of an intrathecal bolus. Once the catheter has been cleared, radiographic contrast can be injected and the couse of the catheter examined along its entire length to detect any dislodgement or leaks. When the catheter is in proper position within the thecal sac, contrast will accumulate along the inner borders of the thecal sac producing a typical lumbar myelogram. Following the side port study, the pump must be carefully programmed to deliver a precise bolus in order to refill the catheter with drug and prevent a period during which no drug is being delivered.
Infectious Complications
Infectious complications are uncommon and can occur within the neuraxis, along the course of the catheter or within the subcutaneous pump pocket. Infections within the neuraxis directly related to intrathecal dug delivery include meningitis and direct infection of the spinal cord near the catheter tip resulting in transverse myelitis.15 Infection involving the implanted pump or catheter can result in the need to remove the device. The incidence of wound infection ranges from 0% to 4.5%, although higher rates of infection have been reported.16 In early superficial infections, the diagnosis can be confused with seroma or hygroma. This is sometimes difficult because each of these complications can involve some redness, edema, and fluctuance of the pocket. The presence of a fever, elevated white blood count, elevated C-reactive protein, and elevated erythrocyte sedimentation rate raises the level of suspicion for an infectious process. In immunocompromised patients, these laboratory values may not change. In cases where purulent discharge with or without wound dehiscence develop, the diagnosis presents no dilemma. In all cases where infection is suspected, culture and Gram stain are helpful to verify the presence of infection and identify the causative organism. In cases where fever and malaise are present but no obvious device infection is found, other causes of infection should be sought.
Device-related Complications
Device-related complications typically occur distant from the original time of implantation and arise from problems with the catheter or the implanted pump. The most common complications are those associated with the intrathecal catheter, but problems with the pump and the subcutaneous pocket and course of the catheter may also arise. Follet and Naumann10 reported a 9.7% rate of catheter-related complications in the first nine months after implantation. The most common complication was that of catheter dislodgement from the intrathecal space, and this study found that more than half of these dislodgements occurred in those where the catheter had not been anchored to the paraspinous fascia. The appearance of new kinks or breaks in the catheter occured with less frequency. A single case where the catheter had been perforated by the needle at the time of a pump refill was reported in the same series.
Migration of the catheter completely out of the spinal canal and into the subcutaneous tissues in the paraspinous region is most common. Migration to the subdural compartment12 or the epidural space has also been described. In all three locations, a loss of the effectiveness of analgesia is likely to be the presenting symptom. It has been hypothesized that subdural catheter migration can also create a loculated region containing high concentrations of the infused drug between the dura mater and the arachnoid mater. This pocket is contained only by the fragile arachnoid membrane, and sudden rupture and release of the loculated drug into the intrathecal space could cause a sudden overdose. Migration can occur into the foramen or toward the nerve root, causing radicular pain or sciatica.17 Reports of catheter migration in to the spinal cord have also appeared18; however, it remains unclear in these cases if the catheter migrated or was actually placed into the cord at the time of implantation, only for symptoms to appear later.14,18 Catheter placement or migration into the spinal cord can go undiagnosed until significant neurologic deficits appear; as with any suspected intrinsic injury to the spinal cord, imaging with MRI is the best means to establish a definitive diagnosis. When catheter migration is suspected, simple radiographic inspection using fluoroscopy, x-ray, or CT as described previously (see Device-related Complications) is the best means to establish the diagnosis.
In patients with indwelling pumps, a refill is required at intervals ranging from 14 to 120 days. During each refill, the pump reservoir must be accessed percutaneously, and there is a risk of inadvertent subcutaneous placement of the drug as well as device or intrathecal infection. Because high concentrations of drug are used and a large dose of the drug will be quickly absorbed if placed subcutaneously, this problem typically becomes life threatening within minutes. The risk of inadvertent subcutaneous placement can be minimized by ensuring that only those with adequate training and familiarity with the pump perform refills. Nonetheless, accessing the pump can be difficult, particularly in those who are obese and when the pump has been placed within a deeper plane. Use of radiographic guidance can simplify identifying the reservoir port (Fig. 25-6). The risk of infection is minimized by appropriate sterile technique, use of a bacteriostatic filter, and the antibacterial effects of any local anesthetics included in the infused agent. Despite the need to frequently perform this procedure, the infection rates appear to be low and should occur in <1% of all refills.19 Drug overdose and device infection have also been reported after attempts to access the port and remove the drug by unauthorized persons. In one case, the patient was trying to gain access to and sell the drug in the pump and intravenous injection of the drug resulted in overdose.20
In most patients, the implanted device causes an inflammatory reaction, and the body creates a fibrous capsule around the device. This fibrous capsule holds the device firmly in position. In malnourished patients, the device can erode through the subcutaneous tissue and other structures. Tissue breakdown can also lead to disastrous erosion into an artery or vein.21
The noninfectious buildup of serosanguineous fluid in the pocket can lead to a seroma that may impede the ability of the wound to heal (see Chapter 26). This can lead to pain, wound breakdown, and dehiscence. Seroma is usually diagnosed by the appearance of a painful erythematous fluctuant mass surrounding the implanted device accompanied by normal laboratory values, lack of fever, and lack of night sweats. Diagnosis is confirmed by aspiration of straw-colored fluid that does not show bacteria on microscopic analysis or subsequent culture. Long-term skin breakdown can occur because of fat necrosis of the subcutaneous tissue. This often accompanies a significant change in weight, most commonly a drop in body mass index.
Intrathecal catheters can develop fibrosis around the catheter tip that results in difficulty aspirating or injecting through the catheter side port. The extent of fibrosis is variable. Minor fibrosis at the catheter tip can go unnoticed, causing problems only when trying to aspirate CSF through the side port. More extensive fibrosis can lead to dangerous inflammatory masses. The occurrence of inflammatory masses surrounding the tip of intrathecal catheters during long-term intrathecal infusion of morphine was first reported in 1999, and, in more recent years, the number of reports and agents associated with inflammatory masses has grown. The inflammatory mass appears to be a chronic fibrotic, noninfectious mass that develops at the tip of the intrathecal catheter over the course of months or years. As the inflammatory mass grows larger, patients often present with neurologic signs and symptoms that reflect direct compression of the spinal cord or other neural elements by the expanding mass.11 Theories regarding the process that leads to inflammatory mass formation include mast cell degranulation creating inflammation of the surrounding tissue. The mast cell theory is well supported by work in animal models, including dogs and sheep.22 Other theories include a nitric oxide response, allergic response, or a reaction to the foreign body of the catheter material, but have little support in the literature. These reactions are a result of the local response to high concentrations of opioid at the catheter tip. Reported cases have been most directly linked to high concentrations of morphine and hydromorphone, although similar granulomas have now been reported with baclofen alone23 as well as combination therapy that included clonidine.24 A consensus statement by a panel of experts recommended that the concentration of morphine be limited to 30 mg/mL and that the concentration of hydromorphone be limited to 20 mg/mL. Because of the reported cases with these two drugs, new interest has arisen in using smaller and more lipid-soluble molecules such as fentanyl or sufentanil. The current literature is limited regarding complications arising from these drugs. The normal course of presentation of an inflammatory mass is an initial loss of pain relief, followed by progressive neurologic loss. The described neurologic symptoms include loss of proprioception, referred pain at the level of the catheter tip, change in sensation, motor loss, and eventually bladder and bowel symptoms. If not diagnosed and treated, this process can progress to paraplegia.11,25 Diagnosis is based on physician suspicion of this complication (Box 25-2). The gold standard for diagnosis is a T1-weighted MRI with and without gadolinium (Fig. 25-7). If the patient cannot undergo an MRI, the second choice for diagnosis is a CT myelogram. The radiologist may require education if not familiar with the appearance of these lesions.
BOX 25-2 Diagnosis of Inflammatory Mass Formation During Long-term Intrathecal Drug Delivery
 Maintain a high of suspicion.
Maintain a high of suspicion.
 Suspect when pain control has been adequate but is suddenly lost.
Suspect when pain control has been adequate but is suddenly lost.
 Suspect when any new neurologic signs or symptoms appear,including new onset o pain,loss of sensation,or weakness in the trunk or lower extremities,as well as new onset of urinary retention or bowel or bladder incontinence.
Suspect when any new neurologic signs or symptoms appear,including new onset o pain,loss of sensation,or weakness in the trunk or lower extremities,as well as new onset of urinary retention or bowel or bladder incontinence.
 The diagnostic study of choice is MRI with gadolinium enhancement.
The diagnostic study of choice is MRI with gadolinium enhancement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


