Key Concepts
 Pain may be classified according to pathophysiology (eg, nociceptive or neuropathic pain), etiology (eg, postoperative or cancer pain), or the affected area (eg, headache or low back pain).
Pain may be classified according to pathophysiology (eg, nociceptive or neuropathic pain), etiology (eg, postoperative or cancer pain), or the affected area (eg, headache or low back pain).
 Nociceptive pain is caused by activation or sensitization of peripheral nociceptors, specialized receptors that transduce noxious stimuli. Neuropathic pain is the result of injury or acquired abnormalities of peripheral or central neural structures.
Nociceptive pain is caused by activation or sensitization of peripheral nociceptors, specialized receptors that transduce noxious stimuli. Neuropathic pain is the result of injury or acquired abnormalities of peripheral or central neural structures.
 Acute pain is caused by noxious stimulation due to injury, a disease process, or the abnormal function of muscle or viscera. It is nearly always nociceptive.
Acute pain is caused by noxious stimulation due to injury, a disease process, or the abnormal function of muscle or viscera. It is nearly always nociceptive.
 Chronic pain is pain that persists beyond the usual course of an acute disease or after a reasonable time for healing to occur; this healing period can vary from 1 to 6 months. Chronic pain may be nociceptive, neuropathic, or mixed.
Chronic pain is pain that persists beyond the usual course of an acute disease or after a reasonable time for healing to occur; this healing period can vary from 1 to 6 months. Chronic pain may be nociceptive, neuropathic, or mixed.
 Modulation of pain occurs peripherally at the nociceptor, in the spinal cord, or in supraspinal structures. This modulation can either inhibit (suppress) or facilitate (intensify) pain.
Modulation of pain occurs peripherally at the nociceptor, in the spinal cord, or in supraspinal structures. This modulation can either inhibit (suppress) or facilitate (intensify) pain.
 At least three mechanisms are responsible for central sensitization in the spinal cord: (1) wind-up and sensitization of second-order wide dynamic range neurons; (2) dorsal horn neuron receptor field expansion; and (3) hyperexitability of flexion reflexes.
At least three mechanisms are responsible for central sensitization in the spinal cord: (1) wind-up and sensitization of second-order wide dynamic range neurons; (2) dorsal horn neuron receptor field expansion; and (3) hyperexitability of flexion reflexes.
 Chronic pain may be caused by a combination of peripheral, central, and psychological mechanisms.
Chronic pain may be caused by a combination of peripheral, central, and psychological mechanisms.
 Moderate to severe acute pain, regardless of site, can affect the function of nearly every organ and may adversely influence perioperative morbidity and mortality.
Moderate to severe acute pain, regardless of site, can affect the function of nearly every organ and may adversely influence perioperative morbidity and mortality.
 The evaluation of any patient with pain should include several key components. Information about location, onset, and quality of pain, as well as alleviating and exacerbating factors, should be obtained along with a pain history that includes previous therapies and changes in symptoms over time.
The evaluation of any patient with pain should include several key components. Information about location, onset, and quality of pain, as well as alleviating and exacerbating factors, should be obtained along with a pain history that includes previous therapies and changes in symptoms over time.
 Psychological evaluation is useful whenever medical evaluation fails to reveal an apparent cause for pain, pain intensity is disproportionate to disease or injury, or when depression or other psychological issues are apparent.
Psychological evaluation is useful whenever medical evaluation fails to reveal an apparent cause for pain, pain intensity is disproportionate to disease or injury, or when depression or other psychological issues are apparent.
 Myofascial pain syndromes are common disorders characterized by aching muscle pain, muscle spasm, stiffness, weakness, and, occasionally, autonomic dysfunction.
Myofascial pain syndromes are common disorders characterized by aching muscle pain, muscle spasm, stiffness, weakness, and, occasionally, autonomic dysfunction.
 Ninety percent of disc herniations occur at L5-S1 or L4-L5. Symptoms usually develop following flexion injuries or heavy lifting and may be associated with bulging, protrusion, or extrusion of the disc.
Ninety percent of disc herniations occur at L5-S1 or L4-L5. Symptoms usually develop following flexion injuries or heavy lifting and may be associated with bulging, protrusion, or extrusion of the disc.
 Back pain caused by spinal stenosis usually radiates into the buttocks, thighs, and legs. Termed pseudoclaudication or neurogenic claudication, the pain is characteristically worse with exercise and relieved by rest, particularly sitting with the spine flexed.
Back pain caused by spinal stenosis usually radiates into the buttocks, thighs, and legs. Termed pseudoclaudication or neurogenic claudication, the pain is characteristically worse with exercise and relieved by rest, particularly sitting with the spine flexed.
 Diabetic neuropathy is the most common type of neuropathic pain.
Diabetic neuropathy is the most common type of neuropathic pain.
 Complex regional pain syndrome (CRPS) is a neuropathic pain disorder with significant autonomic features that is usually subdivided into two variants: CRPS 1, formerly known as reflex sympathetic dystrophy (RSD), and CRPS 2, formerly known as causalgia. The major difference between the two is the absence or presence, respectively, of documented nerve injury.
Complex regional pain syndrome (CRPS) is a neuropathic pain disorder with significant autonomic features that is usually subdivided into two variants: CRPS 1, formerly known as reflex sympathetic dystrophy (RSD), and CRPS 2, formerly known as causalgia. The major difference between the two is the absence or presence, respectively, of documented nerve injury.
 Trigeminal neuralgia (tic douloureux) is classically unilateral and usually located in the V2 or V3 distribution of the trigeminal nerve. It has an electric shock quality lasting from seconds to minutes at a time and is often provoked by contact with a discrete trigger.
Trigeminal neuralgia (tic douloureux) is classically unilateral and usually located in the V2 or V3 distribution of the trigeminal nerve. It has an electric shock quality lasting from seconds to minutes at a time and is often provoked by contact with a discrete trigger.
 Antidepressants are most useful for patients with neuropathic pain. These medications demonstrate an analgesic effect that occurs at a dose lower than needed for antidepressant activity.
Antidepressants are most useful for patients with neuropathic pain. These medications demonstrate an analgesic effect that occurs at a dose lower than needed for antidepressant activity.
 Anticonvulsant medications are useful for patients with neuropathic pain, especially trigeminal neuralgia and diabetic neuropathy.
Anticonvulsant medications are useful for patients with neuropathic pain, especially trigeminal neuralgia and diabetic neuropathy.
 Patients who experience opioid tolerance require escalating doses of opioid to maintain the same analgesic effect. Physical dependence manifests in opioid withdrawal when the opioid medication is either abruptly discontinued or the dose is abruptly and significantly decreased. Psychological dependence, characterized by behavioral changes focusing on drug craving, is rare in cancer patients.
Patients who experience opioid tolerance require escalating doses of opioid to maintain the same analgesic effect. Physical dependence manifests in opioid withdrawal when the opioid medication is either abruptly discontinued or the dose is abruptly and significantly decreased. Psychological dependence, characterized by behavioral changes focusing on drug craving, is rare in cancer patients.
 Complications of stellate block include intravascular and subarachnoid injection, hematoma, pneumothorax, epidural anesthesia, brachial plexus block, hoarseness due to blockade of the recurrent laryngeal nerve, and, rarely, osteomyelitis or mediastinitis.
Complications of stellate block include intravascular and subarachnoid injection, hematoma, pneumothorax, epidural anesthesia, brachial plexus block, hoarseness due to blockade of the recurrent laryngeal nerve, and, rarely, osteomyelitis or mediastinitis.
 Ganglion impar block is effective for patients with visceral or sympathetically maintained pain in the perineal area.
Ganglion impar block is effective for patients with visceral or sympathetically maintained pain in the perineal area.
 Neurolytic blocks are indicated for patients with severe, intractable cancer pain in whom more conventional therapy proves inadequate or conventional analgesic modalities are accompanied by unacceptable side effects.
Neurolytic blocks are indicated for patients with severe, intractable cancer pain in whom more conventional therapy proves inadequate or conventional analgesic modalities are accompanied by unacceptable side effects.
 Spinal cord stimulation may be most effective for neuropathic pain; accepted indications include sympathetically mediated pain, spinal cord lesions with localized segmental pain, phantom limb pain, ischemic lower extremity pain due to peripheral vascular disease, adhesive arachnoiditis, peripheral neuropathies, post-thoracotomy pain, intercostal neuralgia, postherpetic neuralgia, angina, visceral abdominal pain, and visceral pelvic pain.
Spinal cord stimulation may be most effective for neuropathic pain; accepted indications include sympathetically mediated pain, spinal cord lesions with localized segmental pain, phantom limb pain, ischemic lower extremity pain due to peripheral vascular disease, adhesive arachnoiditis, peripheral neuropathies, post-thoracotomy pain, intercostal neuralgia, postherpetic neuralgia, angina, visceral abdominal pain, and visceral pelvic pain.
 Patients with pathological or osteoporotic vertebral compression fracture may benefit from vertebral augmentation with polymethylmethacrylate cement. Vertebroplasty involves injection of the cement through the trocar needle. Kyphoplasty involves inflation of a balloon inserted through a percutaneously placed trocar needle, with subsequent injection of cement.
Patients with pathological or osteoporotic vertebral compression fracture may benefit from vertebral augmentation with polymethylmethacrylate cement. Vertebroplasty involves injection of the cement through the trocar needle. Kyphoplasty involves inflation of a balloon inserted through a percutaneously placed trocar needle, with subsequent injection of cement.
 Acupuncture can be a useful adjunct for patients with chronic pain, particularly that associated with chronic musculoskeletal disorders and headaches.
Acupuncture can be a useful adjunct for patients with chronic pain, particularly that associated with chronic musculoskeletal disorders and headaches.
Chronic Pain Management: Introduction
Pain—the most common symptom that brings patients to see a physician—is nearly always a manifestation of a pathological process. This symptom may have a wide variety of causes ranging from relatively benign conditions to acute injury, myocardial ischemia, degenerative changes, or malignancy. In most cases, after a diagnosis is made, conservative measures are prescribed and the patient responds successfully. In others, referral to a pain medicine specialist for evaluation and treatment improves outcomes and conserves health care resources. In still other situations, pain persists and patients develop chronic pain, the cause of which remains obscure after preliminary investigations have excluded serious and life-threatening illnesses and, if warranted, surgical intervention has either failed to relieve pain or has produced a new pain syndrome.
The term pain management in a general sense applies to the entire discipline of anesthesiology, but its modern usage more specifically involves management of pain throughout the perioperative period as well as nonsurgical pain in both inpatient and outpatient settings. Pain medicine practice may be broadly divided into acute and chronic pain management. The former primarily deals with patients recovering from surgery or with acute medical conditions in a hospital setting (see Chapter 48), whereas the latter includes diverse groups of patients almost always seen in the outpatient setting. Unfortunately, this distinction is artificial and considerable overlap exists; a good example is the patient with cancer who frequently requires short- and long-term pain management in both inpatient and outpatient settings.
The contemporary practice of pain management is not limited to anesthesiologists but often includes other physicians (physiatrists, surgeons, internists, oncologists, psychiatrists, and neurologists) and nonphysicians (psychologists, physical therapists, acupuncturists, and hypnotists). The most effective approaches are multidisciplinary, in which the patient is evaluated by one or more physicians who conduct an initial examination, make a diagnosis, and formulate a treatment plan, and where subsequent evaluation and use of the services and resources of other health care providers are readily available.
Anesthesiologists trained in pain management are in a unique position to coordinate multidisciplinary pain management centers because of their broad training in dealing with a wide variety of patients from surgical, obstetric, pediatric, and medical subspecialties and their expertise in clinical pharmacology and applied neuroanatomy, including the use of peripheral and central nerve blocks.
Like other conscious sensations, normal pain perception depends on specialized neurons that function as receptors, detecting the stimulus, and then transducing and conducting it to the central nervous system. Sensation is often described as either protopathic (noxious) or epicritic (nonnoxious). Epicritic sensations (light touch, pressure, proprioception, and temperature discrimination) are characterized by low-threshold receptors and are generally conducted by large myelinated nerve fibers. In contrast, protopathic sensations (pain) are detected by high-threshold receptors and conducted by smaller, lightly myelinated (Aδ) and unmyelinated (C) nerve fibers.
Pain is not just a sensory modality but an experience. The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” This definition recognizes the interplay between the objective, physiological sensory aspects of pain and its subjective, emotional, and psychological components. The response to pain can be highly variable among different individuals as well as in the same person at different times.
The term nociception is derived from noci (Latin for harm or injury) and is used to describe neural responses to traumatic or noxious stimuli. All nociception produces pain, but not all pain results from nociception. Many patients experience pain in the absence of noxious stimuli. It is therefore clinically useful to divide pain into one of two categories: (1) acute pain, which is primarily due to nociception, and (2) chronic pain, which may be due to nociception, but in which psychological and behavioral factors often play a major role. Table 47-1 lists terms frequently used in describing pain.
| Term | Description |
|---|---|
| Allodynia | Perception of an ordinarily nonnoxious stimulus as pain |
| Analgesia | Absence of pain perception |
| Anesthesia | Absence of all sensation |
| Anesthesia dolorosa | Pain in an area that lacks sensation |
| Dysesthesia | Unpleasant or abnormal sensation with or without a stimulus |
| Hypalgesia (hypoalgesia) | Diminished response to noxious stimulation (eg, pinprick) |
| Hyperalgesia | Increased response to noxious stimulation |
| Hyperesthesia | Increased response to mild stimulation |
| Hyperpathia | Presence of hyperesthesia, allodynia, and hyperalgesia usually associated with overreaction, and persistence of the sensation after the stimulus |
| Hypesthesia (hypoesthesia) | Reduced cutaneous sensation (eg, light touch, pressure, or temperature) |
| Neuralgia | Pain in the distribution of a nerve or a group of nerves |
| Paresthesia | Abnormal sensation perceived without an apparent stimulus |
| Radiculopathy | Functional abnormality of one or more nerve roots |
 Pain may also be classified according to pathophysiology (eg, nociceptive or neuropathic pain), etiology (eg, arthritis or cancer pain), or the affected area (eg, headache or low back pain). Such classifications are useful in the selection of treatment modalities and drug therapy.
Pain may also be classified according to pathophysiology (eg, nociceptive or neuropathic pain), etiology (eg, arthritis or cancer pain), or the affected area (eg, headache or low back pain). Such classifications are useful in the selection of treatment modalities and drug therapy.  Nociceptive pain is caused by activation or sensitization of peripheral nociceptors, specialized receptors that transduce noxious stimuli. Neuropathic pain is the result of injury or acquired abnormalities of peripheral or central neural structures.
Nociceptive pain is caused by activation or sensitization of peripheral nociceptors, specialized receptors that transduce noxious stimuli. Neuropathic pain is the result of injury or acquired abnormalities of peripheral or central neural structures.
There are differences in pain perception related to gender and age. Research has confirmed differences in pain experiences and coping strategies between genders, and there is ongoing investigation into exactly how this processing differs. Brain activation differs between genders, with men particularly influenced by the type and intensity of a noxious stimulus. Brain imaging patterns differ as well. Some of these differences decrease with age and may disappear after age 40.
 Acute pain is caused by noxious stimulation due to injury, a disease process, or the abnormal function of muscle or viscera. It is usually nociceptive. Nociceptive pain serves to detect, localize, and limit tissue damage. Four physiological processes are involved: transduction, transmission, modulation, and perception. This type of pain is typically associated with a neuroendocrine stress response that is proportional to the pain’s intensity. Its most common forms include post-traumatic, postoperative, and obstetric pain as well as pain associated with acute medical illnesses, such as myocardial infarction, pancreatitis, and renal calculi. Most forms of acute pain are self-limited or resolve with treatment in a few days or weeks. When pain fails to resolve because of either abnormal healing or inadequate treatment, it becomes chronic (below). Two types of acute (nociceptive) pain—somatic and visceral—are differentiated based on origin and features.
Acute pain is caused by noxious stimulation due to injury, a disease process, or the abnormal function of muscle or viscera. It is usually nociceptive. Nociceptive pain serves to detect, localize, and limit tissue damage. Four physiological processes are involved: transduction, transmission, modulation, and perception. This type of pain is typically associated with a neuroendocrine stress response that is proportional to the pain’s intensity. Its most common forms include post-traumatic, postoperative, and obstetric pain as well as pain associated with acute medical illnesses, such as myocardial infarction, pancreatitis, and renal calculi. Most forms of acute pain are self-limited or resolve with treatment in a few days or weeks. When pain fails to resolve because of either abnormal healing or inadequate treatment, it becomes chronic (below). Two types of acute (nociceptive) pain—somatic and visceral—are differentiated based on origin and features.
Somatic pain can be further classified as superficial or deep. Superficial somatic pain is due to nociceptive input arising from skin, subcutaneous tissues, and mucous membranes. It is characteristically well localized and described as a sharp, pricking, throbbing, or burning sensation.
Deep somatic pain arises from muscles, tendons, joints, or bones. In contrast to superficial somatic pain, it usually has a dull, aching quality and is less well localized. An additional feature is that both the intensity and duration of the stimulus affect the degree of localization. For example, pain following brief minor trauma to the elbow joint is localized to the elbow, but severe or sustained trauma often causes pain in the whole arm.
Visceral acute pain is due to a disease process or abnormal function involving an internal organ or its covering (eg, parietal pleura, pericardium, or peritoneum). Four subtypes are described: (1) true localized visceral pain, (2) localized parietal pain, (3) referred visceral pain, and (4) referred parietal pain. True visceral pain is dull, diffuse, and usually midline. It is frequently associated with abnormal sympathetic or parasympathetic activity causing nausea, vomiting, sweating, and changes in blood pressure and heart rate. Parietal pain is typically sharp and often described as a stabbing sensation that is either localized to the area around the organ or referred to a distant site (Table 47-2). The phenomenon of visceral or parietal pain referred to cutaneous areas results from patterns of embryological development and migration of tissues, and the convergence of visceral and somatic afferent input into the central nervous system. Thus, pain associated with disease processes involving the peritoneum or pleura over the central diaphragm is frequently referred to the neck and shoulder, whereas pain from disease processes affecting the parietal surfaces of the peripheral diaphragm is referred to the chest or upper abdominal wall.
| Location | Cutaneous Dermatome |
|---|---|
| Central diaphragm | C4 |
| Lungs | T2-T6 |
| Aorta | T1-L2 |
| Heart | T1-T4 |
| Esophagus | T3-T8 |
| Pancreas and spleen | T5-T10 |
| Stomach, liver, and gallbladder | T6-T9 |
| Adrenals | T8-L1 |
| Small intestine | T9-T11 |
| Colon | T10-L1 |
| Kidney, ovaries, and testes | T10-L1 |
| Ureters | T10-T12 |
| Uterus | T11-L2 |
| Bladder and prostate | S2-S4 |
| Urethra and rectum | S2-S4 |
 Chronic pain is pain that persists beyond the usual course of an acute disease or after a reasonable time for healing to occur; this healing period typically can vary from 1 to 6 months. Chronic pain may be nociceptive, neuropathic, or mixed. A distinguishing feature is that psychological mechanisms or environmental factors frequently play a major role. Patients with chronic pain often have attenuated or absent neuroendocrine stress responses and have prominent sleep and affective (mood) disturbances. Neuropathic pain is classically paroxysmal and lancinating, has a burning quality, and is associated with hyperpathia. When it is also associated with loss of sensory input (eg, amputation) into the central nervous system, it is termed deafferentation pain. When the sympathetic system plays a major role, it is often termed sympathetically maintained pain.
Chronic pain is pain that persists beyond the usual course of an acute disease or after a reasonable time for healing to occur; this healing period typically can vary from 1 to 6 months. Chronic pain may be nociceptive, neuropathic, or mixed. A distinguishing feature is that psychological mechanisms or environmental factors frequently play a major role. Patients with chronic pain often have attenuated or absent neuroendocrine stress responses and have prominent sleep and affective (mood) disturbances. Neuropathic pain is classically paroxysmal and lancinating, has a burning quality, and is associated with hyperpathia. When it is also associated with loss of sensory input (eg, amputation) into the central nervous system, it is termed deafferentation pain. When the sympathetic system plays a major role, it is often termed sympathetically maintained pain.
The most common forms of chronic pain include those associated with musculoskeletal disorders, chronic visceral disorders, lesions of peripheral nerves, nerve roots, or dorsal root ganglia (including diabetic neuropathy, causalgia, phantom limb pain, and postherpetic neuralgia), lesions of the central nervous system (stroke, spinal cord injury, and multiple sclerosis), and cancer pain. The pain of most musculoskeletal disorders (eg, rheumatoid arthritis and osteoarthritis) is primarily nociceptive, whereas pain associated with peripheral or central neural disorders is primarily neuropathic. The pain associated with some disorders, eg, cancer and chronic back pain (particularly after surgery), is often mixed. Some clinicians use the term chronic benign pain when pain does not result from cancer. This terminology should be discouraged, however, because pain is never benign from the patient’s point of view, regardless of its cause.
Anatomy & Physiology of Nociception
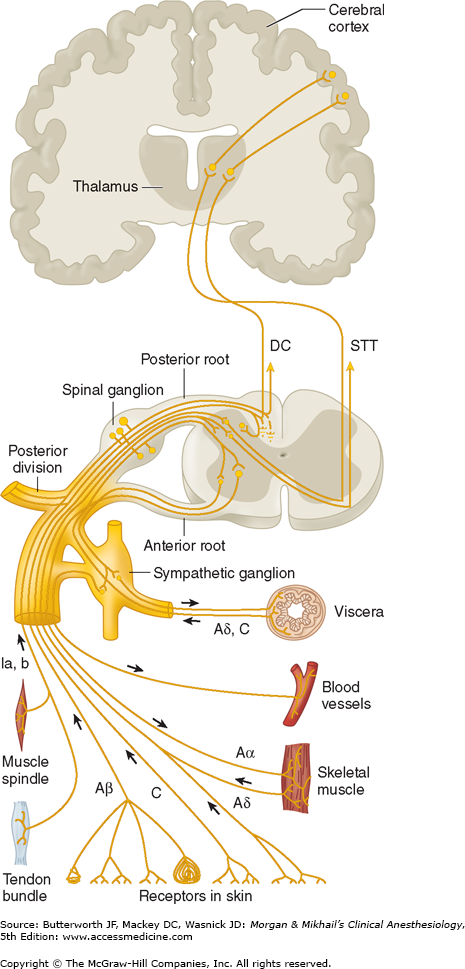
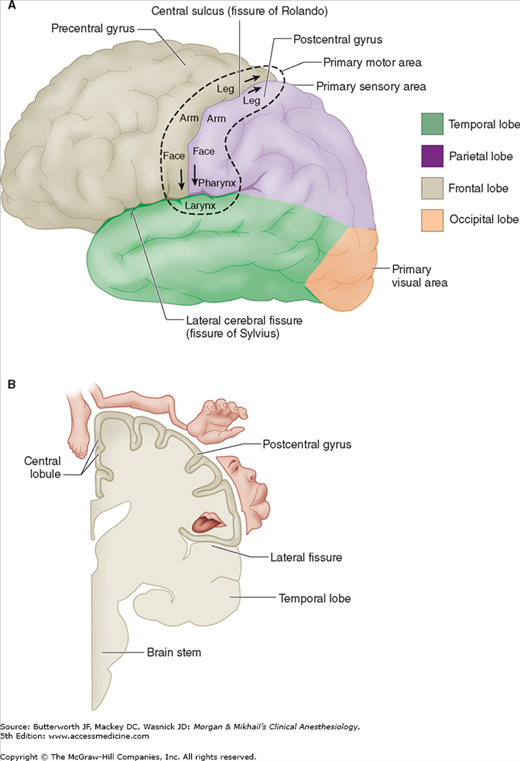
Pain is conducted along three neuronal pathways that transmit noxious stimuli from the periphery to the cerebral cortex (Figure 47-1). The cell bodies of primary afferent neurons are located in the dorsal root ganglia, which lie in the vertebral foramina at each spinal cord level. Each neuron has a single axon that bifurcates, sending one end to the peripheral tissues it innervates and the other into the dorsal horn of the spinal cord. In the dorsal horn, the primary afferent neuron synapses with a second-order neuron whose axon crosses the midline and ascends in the contralateral spinothalamic tract to reach the thalamus. Second-order neurons synapse in thalamic nuclei with third-order neurons, which in turn send projections through the internal capsule and corona radiata to the postcentral gyrus of the cerebral cortex (Figure 47-2).
The majority of first-order neurons send the proximal end of their axons into the spinal cord via the dorsal (sensory) spinal root at each cervical, thoracic, lumbar, and sacral level. Some unmyelinated afferent (C) fibers have been shown to enter the spinal cord via the ventral nerve (motor) root, accounting for observations that some patients continue to feel pain even after transection of the dorsal nerve root (rhizotomy) and report pain following ventral root stimulation. Once in the dorsal horn, in addition to synapsing with second-order neurons, the axons of first-order neurons may synapse with interneurons, sympathetic neurons, and ventral horn motor neurons.
Pain fibers originating from the head are carried by the trigeminal (V), facial (VII), glossopharyngeal (IX), and vagal (X) nerves. The gasserian ganglion contains the cell bodies of sensory fibers in the ophthalmic, maxillary, and mandibular divisions of the trigeminal nerve. Cell bodies of first-order afferent neurons of the facial nerve are located in the geniculate ganglion; those of the glossopharyngeal nerve lie in its superior and petrosal ganglia; and those of the vagal nerve are located in the jugular ganglion (somatic) and the ganglion nodosum (visceral). The proximal axonal processes of the first-order neurons in these ganglia reach the brainstem nuclei via their respective cranial nerves, where they synapse with second-order neurons in brainstem nuclei.
As afferent fibers enter the spinal cord, they segregate according to size, with large, myelinated fibers becoming medial, and small, unmyelinated fibers becoming lateral. Pain fibers may ascend or descend one to three spinal cord segments in Lissauer’s tract before synapsing with second-order neurons in the gray matter of the ipsilateral dorsal horn. In many instances they communicate with second-order neurons through interneurons.
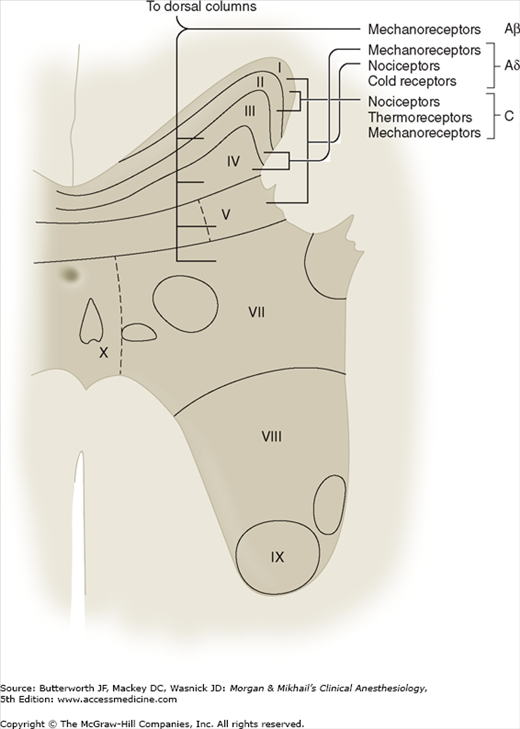
Spinal cord gray matter was divided by Rexed into 10 laminae (Figure 47-3 and Table 47-3). The first six laminae, which make up the dorsal horn, receive all afferent neural activity and represent the principal site of modulation of pain by ascending and descending neural pathways. Second-order neurons are either nociceptive-specific or wide dynamic range (WDR) neurons. Nociceptive-specific neurons serve only noxious stimuli, but WDR neurons also receive nonnoxious afferent input from Aβ, Aδ, and C fibers. Nociceptive-specific neurons are arranged somatotopically in lamina I and have discrete, somatic receptive fields; they are normally silent and respond only to high-threshold noxious stimulation, poorly encoding stimulus intensity. WDR neurons are the most prevalent cell type in the dorsal horn. Although they are found throughout the dorsal horn, WDR neurons are most abundant in lamina V. During repeated stimulation, WDR neurons characteristically increase their firing rate exponentially in a graded fashion (“wind-up”), even with the same stimulus intensity. They also have large receptive fields compared with nociceptive-specific neurons.
| Lamina | Predominant Function | Input | Name |
|---|---|---|---|
| I | Somatic nociception thermoreception | Aδ, C | Marginal layer |
| II | Somatic nociception thermoreception | C, Aδ | Substantia gelatinosa |
| III | Somatic mechanoreception | Aβ, Aδ | Nucleus proprius |
| IV | Mechanoreception | Aβ, Aδ | Nucleus proprius |
| V | Visceral and somatic nociception and mechanoreception | Aβ, Aδ, (C) | Nucleus proprius WDR neurons1 |
| VI | Mechanoreception | Aβ | Nucleus proprius |
| VII | Sympathetic | Intermediolateral column | |
| VIII | Aβ | Motor horn | |
| IX | Motor | Aβ | Motor horn |
| X | Aβ, (Aδ) | Central canal |
Most nociceptive C fibers send collaterals to, or terminate on, second-order neurons in laminae I and II, and, to a lesser extent, in lamina V. In contrast, nociceptive Aδ fibers synapse mainly in laminae I and V, and, to a lesser degree, in lamina X. Lamina I responds primarily to noxious (nociceptive) stimuli from cutaneous and deep somatic tissues. Lamina II, also called the substantia gelatinosa, contains many interneurons and is believed to play a major role in processing and modulating nociceptive input from cutaneous nociceptors. It is also of special interest because it is believed to be a major site of action for opioids. Laminae III and IV primarily receive nonnociceptive sensory input. Laminae VIII and IX make up the anterior (motor) horn. Lamina VII is the intermediolateral column and contains the cell bodies of preganglionic sympathetic neurons.
Visceral afferents terminate primarily in lamina V, and, to a lesser extent, in lamina I. These two laminae represent points of central convergence between somatic and visceral inputs. Lamina V responds to both noxious and nonnoxious sensory input and receives both visceral and somatic pain afferents. The phenomenon of convergence between visceral and somatic sensory input is manifested clinically as referred pain (see Table 47-2). Compared with somatic fibers, visceral nociceptive fibers are fewer in number, more widely distributed, proportionately activate a larger number of spinal neurons, and are not organized somatotopically.
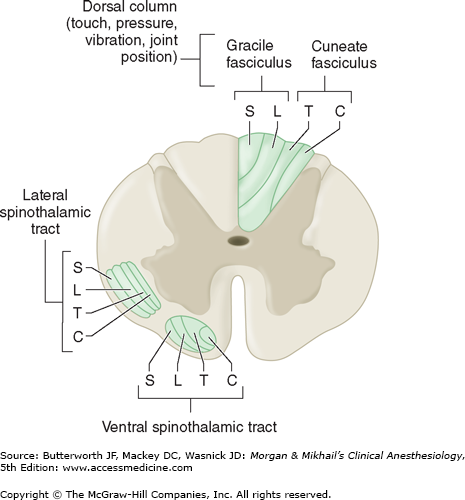
The axons of most second-order neurons cross the midline close to their dermatomal level of origin (at the anterior commissure) to the contralateral side of the spinal cord before they form the spinothalamic tract and send their fibers to the thalamus, the reticular formation, the nucleus raphe magnus, and the periaqueductal gray. The spinothalamic tract, which is classically considered the major pain pathway, lies anterolaterally in the white matter of the spinal cord (Figure 47-4). This ascending tract can be divided into a lateral and a medial tract. The lateral spinothalamic (neospinothalamic) tract projects mainly to the ventral posterolateral nucleus of the thalamus and carries discriminative aspects of pain, such as location, intensity, and duration. The medial spinothalamic (paleospinothalamic) tract projects to the medial thalamus and is responsible for mediating the autonomic and unpleasant emotional perceptions of pain. Some spinothalamic fibers also project to the periaqueductal gray and thus may be an important link between the ascending and descending pathways. Collateral fibers also project to the reticular activating system and the hypothalamus; these are likely responsible for the arousal response to pain.
As with epicritic sensation, pain fibers ascend diffusely, ipsilaterally, and contralaterally; some patients continue to perceive pain following ablation of the contralateral spinothalamic tract, and therefore other ascending pain pathways are also important. The spinoreticular tract is thought to mediate arousal and autonomic responses to pain. The spinomesencephalic tract may be important in activating antinociceptive, descending pathways, because it has some projections to the periaqueductal gray. The spinohypothalamic and spinotelencephalic tracts activate the hypothalamus and evoke emotional behavior. The spinocervical tract ascends uncrossed to the lateral cervical nucleus, which relays the fibers to the contralateral thalamus; this tract is likely a major alternative pathway for pain. Lastly, some fibers in the dorsal columns (which mainly carry light touch and proprioception) are responsive to pain; they ascend medially and ipsilaterally.
Somatic and visceral afferents are fully integrated with the skeletal motor and sympathetic systems in the spinal cord, brainstem, and higher centers. Afferent dorsal horn neurons synapse both directly and indirectly with anterior horn motor neurons. These synapses are responsible for the reflex muscle activity—whether normal or abnormal—that is associated with pain. In a similar fashion, synapses between afferent nociceptive neurons and sympathetic neurons in the intermediolateral column result in reflex sympathetically mediated vasoconstriction, smooth muscle spasm, and the release of catecholamines, both locally and from the adrenal medulla.
Third-order neurons are located in the thalamus and send fibers to somatosensory areas I and II in the postcentral gyrus of the parietal cortex and the superior wall of the sylvian fissure, respectively. Perception and discrete localization of pain take place in these cortical areas. Although most neurons from the lateral thalamic nuclei project to the primary somatosensory cortex, neurons from the intralaminar and medial nuclei project to the anterior cingulate gyrus and are likely involved in mediating the suffering and emotional components of pain.
Nociceptors are characterized by a high threshold for activation and encode the intensity of stimulation by increasing their discharge rates in a graded fashion. Following repeated stimulation, they characteristically display delayed adaptation, sensitization, and afterdischarges.
Noxious sensations can often be broken down into two components: a fast, sharp, and well-localized sensation (“first pain”), which is conducted with a short latency (0.1 s) by Aδ fibers (tested by pinprick); and a slower onset, duller, and often poorly localized sensation (“second pain”), which is conducted by C fibers. In contrast to epicritic sensation, which may be transduced by specialized end organs on the afferent neuron (eg, pacinian corpuscle for touch), protopathic sensation is transduced mainly by free nerve endings.
Most nociceptors are free nerve endings that sense heat and mechanical and chemical tissue damage. Types include (1) mechanonociceptors, which respond to pinch and pinprick, (2) silent nociceptors, which respond only in the presence of inflammation, and (3) polymodal mechanoheat nociceptors. The last are most prevalent and respond to excessive pressure, extremes of temperature (>42°C and <40°C), and noxious substances such as bradykinin, histamine, serotonin (5-hydroxytryptamine or 5-HT), H+, K+, some prostaglandins, capsaicin, and possibly adenosine triphosphate. At least two nociceptor receptors (containing ion channels in nerve endings) have been identified, TRPV1 and TRPV2. Both respond to high temperatures. Capsaicin stimulates the TRPV1 receptor. Polymodal nociceptors are slow to adapt to strong pressure and display heat sensitization.
Nociceptors are present in both somatic and visceral tissues. Primary afferent neurons reach tissues by traveling along spinal somatic, sympathetic, or parasympathetic nerves. Somatic nociceptors include those in skin (cutaneous) and deep tissues (muscle, tendons, fascia, and bone), whereas visceral nociceptors include those in internal organs. The cornea and tooth pulp are unique in that they are almost exclusively innervated by nociceptive Aδ and C fibers.
Deep somatic nociceptors are less sensitive to noxious stimuli than cutaneous nociceptors but are easily sensitized by inflammation. The pain arising from them is characteristically dull and poorly localized. Specific nociceptors exist in muscles and joint capsules, and they respond to mechanical, thermal, and chemical stimuli.
Visceral organs are generally insensitive tissues that mostly contain silent nociceptors. Some organs appear to have specific nociceptors, such as the heart, lung, testis, and bile ducts. Most other organs, such as the intestines, are innervated by polymodal nociceptors that respond to smooth muscle spasm, ischemia, and inflammation. These receptors generally do not respond to the cutting, burning, or crushing that occurs during surgery. A few organs, such as the brain, lack nociceptors altogether; however, the brain’s meningeal coverings do contain nociceptors.
Like somatic nociceptors, those in the viscera are the free nerve endings of primary afferent neurons whose cell bodies lie in the dorsal horn. These afferent nerve fibers, however, frequently travel with efferent sympathetic nerve fibers to reach the viscera. Afferent activity from these neurons enters the spinal cord between T1 and L2. Nociceptive C fibers from the esophagus, larynx, and trachea travel with the vagus nerve to enter the nucleus solitarius in the brainstem. Afferent pain fibers from the bladder, prostate, rectum, cervix and urethra, and genitalia are transmitted into the spinal cord via parasympathetic nerves at the level of the S2-S4 nerve roots. Though relatively few compared with somatic pain fibers, fibers from primary visceral afferent neurons enter the cord and synapse more diffusely with single fibers, often synapsing with multiple dermatomal levels and often crossing to the contralateral dorsal horn.
Several neuropeptides and excitatory amino acids function as neurotransmitters for afferent neurons subserving pain (Table 47-4). Many, if not most, of these neurons contain more than one neurotransmitter, which are simultaneously released. The most important of these peptides are substance P and calcitonin gene-related peptide (CGRP). Glutamate is the most important excitatory amino acid.
| Neurotransmitter | Receptor1 | Effect on Nociception |
|---|---|---|
| Substance P | Neurokinin-1 | Excitatory |
| Calcitonin gene-related peptide | Excitatory | |
| Glutamate | NMDA, AMPA, kainate, quisqualate | Excitatory |
| Aspartate | NMDA, AMPA, kainate, quisqualate | Excitatory |
| Adenosine triphosphate (ATP) | P1, P2 | Excitatory |
| Somatostatin | Inhibitory | |
| Acetylcholine | Muscarinic | Inhibitory |
| Enkephalins | μ, δ, κ | Inhibitory |
| β-Endorphin | μ, δ, κ | Inhibitory |
| Norepinephrine | α2 | Inhibitory |
| Adenosine | A1 | Inhibitory |
| Serotonin | 5-HT1 (5-HT3) | Inhibitory |
| γ-Aminobutyric acid (GABA) | A, B | Inhibitory |
| Glycine | Inhibitory |
Substance P is an 11 amino acid peptide that is synthesized and released by first-order neurons both peripherally and in the dorsal horn. Also found in other parts of the nervous system and the intestines, it facilitates transmission in pain pathways via neurokinin-1 receptor activation. In the periphery, substance P neurons send collaterals that are closely associated with blood vessels, sweat glands, hair follicles, and mast cells in the dermis. Substance P sensitizes nociceptors, degranulates histamine from mast cells and 5-HT from platelets, and is a potent vasodilator and chemoattractant for leukocytes. Substance P-releasing neurons also innervate the viscera and send collateral fibers to paravertebral sympathetic ganglia; intense stimulation of viscera, therefore, can cause direct postganglionic sympathetic discharge.
Both opioid and α2-adrenergic receptors have been described on or near the terminals of unmyelinated peripheral nerves. Although their physiological role is not clear, the latter may explain the observed analgesia of peripherally applied opioids, particularly in the presence of inflammation.
 Modulation of pain occurs peripherally at the nociceptor, in the spinal cord, and in supraspinal structures. This modulation can either inhibit (suppress) or facilitate (intensify) pain.
Modulation of pain occurs peripherally at the nociceptor, in the spinal cord, and in supraspinal structures. This modulation can either inhibit (suppress) or facilitate (intensify) pain.
Nociceptors and their neurons display sensitization following repeated stimulation. Sensitization may be manifested as an enhanced response to noxious stimulation or a newly acquired responsiveness to a wider range of stimuli, including nonnoxious stimuli.
Sensitization of nociceptors results in a decrease in threshold, an increase in the frequency response to the same stimulus intensity, a decrease in response latency, and spontaneous firing even after cessation of the stimulus (afterdischarges). Such sensitization commonly occurs with injury and following application of heat. Primary hyperalgesia is mediated by the release of noxious substances from damaged tissues. Histamine is released from mast cells, basophils, and platelets, whereas serotonin is released from mast cells and platelets. Bradykinin is released from tissues following activation of factor XII. Bradykinin activates free nerve endings via specific B1 and B2 receptors.
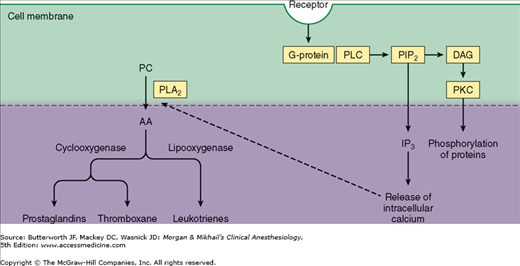
Prostaglandins are produced following tissue damage by the action of phospholipase A2 on phospholipids released from cell membranes to form arachidonic acid (Figure 47-5). The cyclooxygenase (COX) pathway then converts the latter into endoperoxides, which in turn are transformed into prostacyclin and prostaglandin E2 (PGE2). PGE2 directly activates free nerve endings, whereas prostacyclin potentiates the edema from bradykinin. The lipoxygenase pathway converts arachidonic acid into hydroperoxy compounds, which are subsequently converted into leukotrienes. The role of the latter is not well defined, but they appear to potentiate certain types of pain. Pharmacological agents such as acetylsalicylic acid (ASA, or aspirin), acetaminophen, and nonsteroidal antiinflammatory drugs (NSAIDs) produce analgesia by inhibition of COX. The analgesic effect of corticosteroids is likely the result of inhibition of prostaglandin production through blockade of phospholipase A2 activation.
Figure 47-5
Phospholipase C (PLC) catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to produce inositol triphosphate (IP3) and diacylglycerol (DAG). Protein kinase C (PKC) is also important. Phospholipase A2 (PLA2) catalyzes the conversion of phosphatidylcholine (PC) to arachidonic acid (AA).
Neurogenic inflammation, also called secondary hyperalgesia, plays an important role in peripheral sensitization following injury. It is manifested by the “triple response (of Lewis)” of a red flush around the site of injury (flare), local tissue edema, and sensitization to noxious stimuli. Secondary hyperalgesia is primarily due to antidromic release of substance P (and probably CGRP). Substance P degranulates histamine and 5-HT, vasodilates blood vessels, causes tissue edema, and induces the formation of leukotrienes. The neural origin of this response is supported by the following findings: (1) it can be produced by electrical stimulation of a sensory nerve, (2) it is not observed in denervated skin, and (3) it is diminished by injection of a local anesthetic. Capsaicin applied topically in a gel, cream, or patch depletes substance P and diminishes neurogenic inflammation, and is useful for some patients with postherpetic neuralgia.
 At least three mechanisms are responsible for central sensitization in the spinal cord:
At least three mechanisms are responsible for central sensitization in the spinal cord:
Wind-up and sensitization of second-order neurons. WDR neurons increase their frequency of discharge with the same repetitive stimuli and exhibit prolonged discharge, even after afferent C fiber input has stopped.
Receptor field expansion. Dorsal horn neurons increase their receptive fields such that adjacent neurons become responsive to stimuli (whether noxious or not) to which they were previously unresponsive.
Hyperexcitability of flexion reflexes. Enhancement of flexion reflexes is observed both ipsilaterally and contralaterally.
Neurochemical mediators of central sensitization include substance P, CGRP, vasoactive intestinal peptide (VIP), cholecystokinin (CCK), angiotensin, and galanin, as well as the excitatory amino acids L-glutamate and L-aspartate. These substances trigger changes in membrane excitability by interacting with G protein-coupled membrane receptors on neurons (Figure 47-5).
Glutamate and aspartate play important roles in wind-up, via activation of N-methyl-D-aspartate (NMDA) and other receptor mechanisms, and in the induction and maintenance of central sensitization. Activation of NMDA receptors also induces nitric oxide synthetase, increasing formation of nitric oxide. Both prostaglandins and nitric oxide facilitate the release of excitatory amino acids in the spinal cord. Thus, COX inhibitors such as ASA and NSAIDs have important analgesic actions in the spinal cord.
Transmission of nociceptive input in the spinal cord can be inhibited by segmental activity in the cord itself, as well as by descending neural activity from supraspinal centers.
Activation of large afferent fibers subserving sensation inhibits WDR neuron and spinothalamic tract activity. Moreover, activation of noxious stimuli in noncontiguous parts of the body inhibits WDR neurons at other levels, which may explain why pain in one part of the body inhibits pain in other parts. These two phenomena support a “gate” theory for pain processing in the spinal cord.
Glycine and γ-aminobutyric acid (GABA) are amino acids that function as inhibitory neurotransmitters and likely play an important role in segmental inhibition of pain in the spinal cord. Antagonism of glycine and GABA results in powerful facilitation of WDR neurons and produces allodynia and hyperesthesia. There are two subtypes of GABA receptors: GABAA, of which muscimol is an agonist, and GABAB, of which baclofen is an agonist. Segmental inhibition appears to be mediated by GABAB receptor activity. The GABAA receptor functions as a Cl− channel, and benzodiazepines activate this channel. Activation of glycine receptors also increases Cl− conductance across neuronal cell membranes. The action of glycine is more complex than that of GABA, because the former also has a facilitatory (excitatory) effect on the NMDA receptor.
Adenosine also modulates nociceptive activity in the dorsal horn. At least two receptors are known: A1, which inhibits adenyl cyclase, and A2, which stimulates adenyl cyclase. The A1 receptor mediates adenosine’s antinociceptive action. Methylxanthines can reverse this effect through phosphodiesterase inhibition.
Several supraspinal structures send fibers down the spinal cord to inhibit pain in the dorsal horn. Important sites of origin for these descending pathways include the periaqueductal gray, reticular formation, and nucleus raphe magnus (NRM). Stimulation of the periaqueductal gray area in the midbrain produces widespread analgesia in humans. Axons from these tracts act presynaptically on primary afferent neurons and postsynaptically on second-order neurons (or interneurons). These pathways mediate their antinociceptive action via α2-adrenergic, serotonergic, and opiate (μ, δ, and κ) receptor mechanisms. The role of monoamines in pain inhibition explains the analgesic efficacy of antidepressants that block reuptake of catecholamines and serotonin.
Inhibitory adrenergic pathways originate primarily from the periaqueductal gray area and the reticular formation. Norepinephrine mediates this action via activation of presynaptic or postsynaptic α2 receptors. At least part of the descending inhibition from the periaqueductal gray is relayed first to the NRM and medullary reticular formation; serotonergic fibers from the NRM then relay the inhibition to dorsal horn neurons via the dorsolateral funiculus.
The endogenous opiate system (primarily the NRM and reticular formation) acts via methionine enkephalin, leucine enkephalin, and β-endorphin, all of which are antagonized by naloxone. These opioids act presynaptically to hyperpolarize primary afferent neurons and inhibit the release of substance P; they also appear to cause some postsynaptic inhibition. Exogenous opioids preferentially act postsynaptically on the second-order neurons or interneurons in the substantia gelatinosa.
 Chronic pain may be caused by a combination of peripheral, central, and psychological mechanisms. Sensitization of nociceptors plays a major role in the origin of pain associated with peripheral mechanisms, such as chronic musculoskeletal and visceral disorders.
Chronic pain may be caused by a combination of peripheral, central, and psychological mechanisms. Sensitization of nociceptors plays a major role in the origin of pain associated with peripheral mechanisms, such as chronic musculoskeletal and visceral disorders.
Neuropathic pain involves peripheral-central and central neural mechanisms that are complex and generally associated with partial or complete lesions of peripheral nerves, dorsal root ganglia, nerve roots, or more central structures (Table 47-5). Peripheral mechanisms include spontaneous discharges; sensitization of receptors to mechanical, thermal, and chemical stimuli; and up-regulation of adrenergic receptors. Neural inflammation may also be present. Systemic administration of local anesthetics and anticonvulsants has been shown to suppress the spontaneous firing of sensitized or traumatized neurons. This observation is supported by the efficacy of agents such as lidocaine, mexiletine, and carbamazepine in many patients with neuropathic pain. Central mechanisms include loss of segmental inhibition, wind-up of WDR neurons, spontaneous discharges in deafferentated neurons, and reorganization of neural connections.
| Spontaneous self-sustaining neuronal activity in the primary afferent neuron (such as a neuroma). |
| Marked mechanosensitivity associated with chronic nerve compression. |
| Short-circuits between pain fibers and other types of fibers following demyelination, resulting in activation of nociceptive fibers by nonnoxious stimuli at the site of injury (ephaptic transmission). |
| Functional reorganization of receptive fields in dorsal horn neurons such that sensory input from surrounding intact nerves emphasizes or intensifies any input from the area of injury. |
| Spontaneous electrical activity in dorsal horn cells or thalamic nuclei. |
| Release of segmental inhibition in the spinal cord. |
| Loss of descending inhibitory influences that are dependent on normal sensory input. |
| Lesions of the thalamus or other supraspinal structures. |
The sympathetic nervous system appears to play a major role in some patients with chronic pain. The efficacy of sympathetic nerve blocks in some of these patients supports the concept of sympathetically maintained pain. Painful disorders that often respond to sympathetic blocks include complex regional pain syndrome, deafferentation syndromes due to nerve avulsion or amputations, and postherpetic neuralgia. However, the simplistic theory of heightened sympathetic activity resulting in vasoconstriction, edema, and hyperalgesia fails to account for the warm and erythematous phase observed in some patients. Similarly, clinical and experimental observations do not satisfactorily support the theory of ephaptic transmission between pain fibers and demyelinated sympathetic fibers.
Psychological mechanisms or environmental factors are rarely the sole mechanisms for chronic pain but are commonly seen in combination with other mechanisms (Table 47-6).
| Psychophysiological mechanisms in which emotional factors act as the initiating cause (eg, tension headaches). |
| Learned or operant behavior in which chronic behavior patterns are rewarded (eg, by attention of a spouse) following an often minor injury. |
| Psychopathology such as major affective disorders (depression), schizophrenia, and somatization disorders (conversion hysteria) in which the patient has an abnormal preoccupation with bodily functions. |
| Pure psychogenic mechanisms (somatoform pain disorder), in which suffering is experienced despite absence of nociceptive input. |
Acute pain is typically associated with a neuroendocrine stress response that is proportional to pain intensity. The pain pathways mediating the afferent limb of this response are discussed above. The efferent limb is mediated by the sympathetic nervous and endocrine systems. Sympathetic activation increases efferent sympathetic tone to all viscera and releases catecholamines from the adrenal medulla. The hormonal response results from increased sympathetic tone and from hypothalamically mediated reflexes.  Moderate to severe acute pain, regardless of site, can affect the function of nearly every organ and may adversely affect perioperative morbidity and mortality.
Moderate to severe acute pain, regardless of site, can affect the function of nearly every organ and may adversely affect perioperative morbidity and mortality.
Cardiovascular effects are often prominent and include hypertension, tachycardia, enhanced myocardial irritability, and increased systemic vascular resistance. Cardiac output increases in most normal patients but may decrease in patients with compromised ventricular function. Because of the increase in myocardial oxygen demand, pain can worsen or precipitate myocardial ischemia.
An increase in total body oxygen consumption and carbon dioxide production necessitates a concomitant increase in minute ventilation. The latter increases the work of breathing, particularly in patients with underlying lung disease. Pain due to abdominal or thoracic incisions further compromises pulmonary function because of guarding (splinting). Decreased movement of the chest wall reduces tidal volume and functional residual capacity; this promotes atelectasis, intrapulmonary shunting, hypoxemia, and, less commonly, hypoventilation. Reductions in vital capacity impair coughing and clearing of secretions. Regardless of the pain’s location, prolonged bed rest or immobilization can produce similar changes in pulmonary function.
Enhanced sympathetic tone increases sphincter tone and decreases intestinal and urinary motility, promoting ileus and urinary retention, respectively. Hypersecretion of gastric acid can promote stress ulceration and worsen the consequences of pulmonary aspiration. Nausea, vomiting, and constipation are common.
Stress increases catabolic hormones (catecholamines, cortisol, and glucagon) and decreases anabolic hormones (insulin and testosterone). Patients develop a negative nitrogen balance, carbohydrate intolerance, and increased lipolysis. The increase in cortisol, renin, angiotensin, aldosterone, and antidiuretic hormone results in sodium retention, water retention, and secondary expansion of the extracellular space.
Stress-mediated increases in platelet adhesiveness, reduced fibrinolysis, and hypercoagulability have been reported.
The neuroendocrine stress response produces leukocytosis and has been reported to depress the reticuloendothelial system. The latter predisposes patients to infection. Stress-related immunodepression may also enhance tumor growth and metastasis.
Anxiety and sleep disturbances are common reactions to acute pain. With prolonged duration of the pain, depression is not unusual. Some patients react with frustration and anger that may be directed at family, friends, or the medical staff.
The neuroendocrine stress response is generally observed only in patients with severe recurring pain due to peripheral (nociceptive) mechanisms and in patients with prominent central mechanisms such as pain associated with paraplegia. It is attenuated or absent in most patients with chronic pain. Sleep and affective disturbances, particularly depression, are often prominent. Many patients also experience significant changes in appetite (increase or decrease) and stresses on social relationships.
Evaluation of the Patient with Chronic Pain
 The evaluation of any patient with pain should include several key components. Information about location, onset, and quality of pain, as well as alleviating and exacerbating factors, should be obtained, along with a pain history that includes previous therapies and changes in symptoms over time. In addition to physical symptoms, chronic pain usually involves a psychological component that should be addressed as well. Questionnaires, diagrams, and pain scales are useful tools in helping patients adequately describe the characteristics of their pain and how it affects their quality of life. Information gathered during the physical examination can help distinguish pain location, type, and systemic sequelae, if any. Imaging studies such as plain radiographs, computed tomography (CT), magnetic resonance imaging (MRI), and bone scans can often suggest physiological causes. All components are necessary for a comprehensive evaluation of the pain patient prior to determining appropriate treatment options.
The evaluation of any patient with pain should include several key components. Information about location, onset, and quality of pain, as well as alleviating and exacerbating factors, should be obtained, along with a pain history that includes previous therapies and changes in symptoms over time. In addition to physical symptoms, chronic pain usually involves a psychological component that should be addressed as well. Questionnaires, diagrams, and pain scales are useful tools in helping patients adequately describe the characteristics of their pain and how it affects their quality of life. Information gathered during the physical examination can help distinguish pain location, type, and systemic sequelae, if any. Imaging studies such as plain radiographs, computed tomography (CT), magnetic resonance imaging (MRI), and bone scans can often suggest physiological causes. All components are necessary for a comprehensive evaluation of the pain patient prior to determining appropriate treatment options.
Reliable quantitation of pain severity helps determine therapeutic interventions and evaluate the efficacy of treatments. This is a challenge, however, because pain is a subjective experience that is influenced by psychological, cultural, and other variables. Clear definitions are necessary, because pain may be described in terms of tissue destruction or bodily or emotional reaction.
The numerical rating scale, Wong-Baker FACES rating scale, visual analog scale (VAS), and McGill Pain Questionnaire (MPQ) are most commonly used. In the numerical scale, 0 corresponds to no pain and 10 is intended to reflect the worst possible pain. The Wong-Baker FACES pain scale, designed for children 3 years of age and older, is useful in patients with whom communication may be difficult. The patient is asked to point to various facial expressions ranging from a smiling face (no pain) to an extremely unhappy one that expresses the worst possible pain. The VAS is a 10-cm horizontal line labeled “no pain” at one end and “worst pain imaginable” on the other end. The patient is asked to mark on this line where the intensity of the pain lies. The distance from “no pain” to the patient’s mark numerically quantifies the pain. The VAS is a simple and efficient method that correlates well with other reliable methods.
The MPQ is a checklist of words describing symptoms. Unlike other pain rating methods that assume pain is one-dimensional and describe intensity but not quality, the MPQ attempts to define the pain in three major dimensions: (1) sensory-discriminative (nociceptive pathways), (2) motivational-affective (reticular and limbic structures), and (3) cognitive-evaluative (cerebral cortex). It contains 20 sets of descriptive words that are divided into four major groups: 10 sensory, 5 affective, 1 evaluative, and 4 miscellaneous. The patient selects the sets that apply to his or her pain and circles the words in each set that best describe the pain. The words in each class are given rank according to severity of pain. A pain rating index is derived based on the words chosen.
 Psychological evaluation is useful whenever medical evaluation fails to reveal an apparent cause for pain, when pain intensity, characteristics, or duration are disproportionate to disease or injury, or when depression or other psychological issues are apparent. These types of evaluations help define the role of psychological or behavioral factors. The most commonly used tests are the Minnesota Multiphasic Personality Inventory (MMPI) and Beck Depression Inventory.
Psychological evaluation is useful whenever medical evaluation fails to reveal an apparent cause for pain, when pain intensity, characteristics, or duration are disproportionate to disease or injury, or when depression or other psychological issues are apparent. These types of evaluations help define the role of psychological or behavioral factors. The most commonly used tests are the Minnesota Multiphasic Personality Inventory (MMPI) and Beck Depression Inventory.
The MMPI is a 566-item true-false questionnaire that attempts to define the patient’s personality on 10 clinical scales. Three validity scales serve to identify patients deliberately trying to hide traits or alter the results. Cultural differences can affect scores. Moreover, the test is lengthy and some patients find its questions insulting. The MMPI is used primarily to confirm clinical impressions about the role of psychological factors; it cannot reliably distinguish between “organic” and “functional” pain.
Depression is very common in patients with chronic pain. It is often difficult to determine the relative contribution of depression to the suffering associated with pain. The Beck Depression Inventory is a useful test for identifying patients with major depression.
Several tests have been developed to assess functional limitations or impairment (disability). These include the Multidimensional Pain Inventory (MPI), Medical Outcomes Survey 36-Item Short Form (SF-36), Pain Disability Index (PDI), and Oswestry Disability Index (ODI).
Emotional disorders are commonly associated with complaints of chronic pain, and chronic pain often results in varying degrees of psychological distress. Determination of which came first is often difficult. In either case, both the pain and emotional distress need to be treated. Table 47-7 lists emotional disorders in which treatment should be primarily directed at the emotional disorder.
| Disorder | Brief Description |
|---|---|
| Somatization disorder | Physical symptoms of a medical condition that cannot be explained, resulting in involuntary distress and physical impairment. |
| Conversion disorder | Symptoms of voluntary motor or sensory deficits that suggest a medical condition; symptoms cannot be medically explained but are associated with psychological factors and are not intentionally feigned. |
| Hypochondriasis | Prolonged (>6 months) preoccupation with the fear of having a serious illness despite adequate medical evaluation and reassurance. |
| Malingering | Intentional production of physical or psychological symptoms that is motivated by external incentives (eg, avoiding work or financial compensation). |
| Substance-related disorders | Habitual misuse of prescribed or illicit substances that often precedes and drives complaints of pain and drug-seeking behavior. |
Electromyography and nerve conduction studies complement one another and are useful for confirming the diagnosis of entrapment syndromes, radicular syndromes, neural trauma, and polyneuropathies. They can often distinguish between neurogenic and myogenic disorders. Patterns of abnormalities can localize a lesion to the spinal cord, nerve root, limb plexus, or peripheral nerve. In addition, they may also be useful in excluding “organic” disorders when psychogenic pain or a “functional” syndrome is suspected.
Electromyography employs needle electrodes to record potentials in individual muscles. Muscle potentials are recorded first while the muscle is at rest and then as the patient is asked to move the muscle. A triphasic motor unit action potential is normally seen as the patient voluntarily moves the muscle. Abnormal findings suggestive of denervation include persistent insertion potentials, the presence of positive sharp waves, fibrillary activity, or fasciculation potentials. Abnormalities in muscles produce changes in amplitude and duration as well as polyphasic action potentials.
Peripheral nerve conduction studies employ supramaximal stimulations of motor or mixed sensorimotor nerve, whereas muscle potentials are recorded over the appropriate muscle. The time between the onset of the stimulation and the onset of the muscle potential (latency) is a measurement of the fastest conducting motor fibers in the nerve. The amplitude of the recorded potential indicates the number of functional motor units, whereas its duration reflects the range of conduction velocities in the nerve. Conduction velocity can be obtained by stimulating the nerve from two points and comparing the latencies. When a pure sensory nerve is evaluated, the nerve is stimulated while action potentials are recorded either proximally or distally (antidromic conduction).
Nerve conduction studies distinguish between mononeuropathies (due to trauma, compression, or entrapment) and polyneuropathies. The latter include systemic disorders that may produce abnormalities that are widespread and symmetrical or that are random (eg, mononeuropathy multiplex).
Selected Pain Syndromes
Neural compression may occur wherever a nerve courses through an anatomically narrowed passage, and entrapment neuropathies can involve sensory, motor, or mixed nerves. Genetic factors and repetitive macrotrauma or microtrauma are likely involved, and adjacent tenosynovitis is often responsible. Table 47-8 lists the most commonly recognized entrapment syndromes. When a sensory nerve is involved, patients complain of pain and numbness in its distribution distal to the site of entrapment; occasionally, a patient may complain of pain referred proximal to the site of entrapment. Entrapment of the sciatic nerve can mimic a herniated intervertebral disc. Entrapment of a motor nerve produces weakness in the muscles it innervates. Even entrapments of “pure” motor nerves can produce a vague pain that may be mediated by afferent fibers from muscles and joints. The diagnosis can usually be confirmed by electromyography and nerve conduction studies. Neural blockade of the nerve with local anesthetic, with or without corticosteroid, may be diagnostic and can provide temporary pain relief. Treatment is generally symptomatic with oral analgesics and temporary immobilization, whenever appropriate. Development of complex regional pain syndrome may respond to sympathetic blocks. Refractory symptoms may require surgical decompression.
| Nerve | Entrapment Site | Location of Pain |
|---|---|---|
| Cranial nerves VII, IX, and X | Styloid process or stylohyoid ligament | Ipsilateral tonsil, base of tongue, temporomandibular joint, and ear (Eagle’s syndrome) |
| Brachial plexus | Scalenus anticus muscle or a cervical rib | Ulnar side of arm and forearm (scalenus anticus syndrome) |
| Suprascapular nerve | Suprascapular notch | Posterior and lateral shoulder |
| Median nerve | Pronator teres muscle | Proximal forearm and palmar surface of the first three digits (pronator syndrome) |
| Median nerve | Carpal tunnel | Palmar surface of the first three digits (carpal tunnel syndrome) |
| Ulnar nerve | Cubital fossa (elbow) | Fourth and fifth digits of the hand (cubital tunnel syndrome) |
| Ulnar nerve | Guyon’s canal (wrist) | Fourth and fifth digits of the hand |
| Lateral femoral cutaneous nerve | Anterior iliac spine under the inguinal ligament | Anterolateral thigh (meralgia paresthetica) |
| Obturator nerve | Obturator canal | Upper medial thigh |
| Saphenous nerve | Subsartorial tunnel (adductor canal) | Medial calf |
| Sciatic nerve | Sciatic notch | Buttock and leg (piriformis syndrome) |
| Common peroneal nerve | Fibular neck | Lateral distal leg and foot |
| Deep peroneal nerve | Anterior tarsal tunnel | Big toe or foot |
| Superficial peroneal nerve | Deep fascia above the ankle | Anterior ankle and dorsum of foot |
| Posterior tibial nerve | Posterior tarsal tunnel | Undersurface of foot (tarsal tunnel syndrome) |
| Interdigital nerve | Deep transverse tarsal ligament | Between toes and foot (Morton’s neuroma) |
 Myofascial pain syndromes are common disorders characterized by aching muscle pain, muscle spasm, stiffness, weakness, and, occasionally, autonomic dysfunction. Patients have discrete areas (trigger points) of marked tenderness in one or more muscles or the associated connective tissue. Palpation of the involved muscles may reveal tight, ropy bands over trigger points. Signs of autonomic dysfunction (vasoconstriction or piloerection) in the overlying muscles may be present. The pain characteristically radiates in a fixed pattern that does not follow dermatomes.
Myofascial pain syndromes are common disorders characterized by aching muscle pain, muscle spasm, stiffness, weakness, and, occasionally, autonomic dysfunction. Patients have discrete areas (trigger points) of marked tenderness in one or more muscles or the associated connective tissue. Palpation of the involved muscles may reveal tight, ropy bands over trigger points. Signs of autonomic dysfunction (vasoconstriction or piloerection) in the overlying muscles may be present. The pain characteristically radiates in a fixed pattern that does not follow dermatomes.
Gross trauma or repetitive microtrauma is thought to play a major role in initiating myofascial pain syndromes. Trigger points develop following acute injury; stimulation of these active trigger points produces pain, and the ensuing muscle spasm sustains the pain. When the acute episode subsides, the trigger points become latent (tender, but not pain producing) only to be reactivated at a later time by subsequent stress. The pathophysiology is poorly understood.
The diagnosis of a myofascial pain syndrome is suggested by the character of the pain and by palpation of discrete trigger points that reproduce it. Common syndromes produce trigger points in the levator scapulae, masseter, quadratus lumborum, and gluteus medius muscles. The latter two syndromes produce low back pain and should be considered in all patients with back pain; moreover, gluteal trigger points can mimic S1 radiculopathy.
Although myofascial pain may spontaneously resolve without sequelae, many patients continue to have latent trigger points. When trigger points are active, treatment is directed at regaining muscle length and elasticity. Analgesia may be provided utilizing local anesthetic (1-3 mL) trigger point injections. Topical cooling with either an ethyl chloride or fluorocarbon (fluoromethane) spray can also induce reflex muscle relaxation, facilitating massage (“stretch and spray”) and ultrasound therapy. Physical therapy is important in establishing and maintaining normal range of motion for affected muscles, and biofeedback may be helpful.
The American College of Rheumatology recently identified three criteria that, if met, suggest the diagnosis of fibromyalgia:
Widespread Pain Index (WPI) score of 7 or higher, and Symptom Severity (SS) scale score of 5 or higher, or WPI of 3-6 and SS scale score of 9 or higher.
Symptoms present at a similar level for at least 3 months.
Absence of another disorder that would otherwise explain the pain.
Treatment of fibromyalgia includes cardiovascular conditioning, strength training, improving sleep hygiene, cognitive-behavioral therapy, patient education, and pharmacotherapy. Medications approved by the U.S. Food and Drug Administration (FDA) for the treatment of fibromyalgia include pregabalin (Lyrica), duloxetine (Cymbalta), and milnacipran (Savella).
Back pain is an extremely common complaint and a major cause of work disability worldwide. Lumbosacral strain, degenerative disc disease, and myofascial syndromes are the most common causes. Low back pain, with or without associated leg pain, may also have congenital, traumatic, degenerative, inflammatory, infectious, metabolic, psychological, and neoplastic causes. Moreover, back pain can be due to disease processes in the abdomen and pelvis, particularly those affecting retroperitoneal structures (pancreas, kidneys, ureters, and aorta), the uterus and adnexa, the prostate, and the rectosigmoid colon. Disorders of the hip can also mimic back disorders. A positive Patrick’s sign (or Patrick’s test)—ie, the elicitation of pain in the hip or sacroiliac joint when the examiner places the ipsilateral heel of the supine patient on the contralateral knee and presses down on the ipsilateral knee—helps identify back pain due to hip or sacroiliac joint disorders. This sign is also referred to by an acronym, FABERE (sign), because the movement of the leg involves flexion, abduction, external rotation, and extension.
The back can be described in terms of anterior and posterior elements. The anterior elements consist of cylindrical vertebral bodies interconnected by intervertebral discs and supported by anterior and posterior longitudinal ligaments. The posterior elements are bony arches extending from each vertebral body, consisting of two pedicles, two transverse processes, two laminae, and a spinous process. The transverse and spinous processes provide points of attachment for the muscles that move and protect the spinal column. Adjacent vertebrae also articulate posteriorly by two gliding facet joints.
Spinal structures are innervated by the sinuvertebral branches and posterior rami of spinal nerves. The sinuvertebral nerve arises before each spinal nerve divides into anterior and posterior rami, and reenters the intervertebral foramen to innervate the posterior longitudinal ligament, the posterior annulus fibrosus, periosteum, dura, and epidural vessels. Paraspinal structures are supplied by the posterior primary ramus. Each facet joint is innervated by the medial branch of the posterior primary rami of the spinal nerves above and below the joint.
As lumbar spinal nerve roots exit the dural sac, they travel down 1-2 cm laterally before exiting through their respective intervertebral foramina; thus, for example, the L5 nerve root leaves the dural sac at the level of the L4-L5 disc (where it is more likely to be compressed) but leaves the spinal canal beneath the L5 pedicle opposite the L5-S1 disc.
Approximately 80-90% of low back pain is due to sprain or strain associated with lifting heavy objects, falls, or sudden abnormal movements of the spine. The term sprain is generally used when the pain is related to a well-defined acute injury, whereas strain is used when the pain is more chronic and is likely related to repetitive minor injuries.
Injury to paravertebral muscles and ligaments results in reflex muscle spasm, which may or may not be associated with trigger points. The pain is usually dull and aching, and occasionally radiates down the buttocks or hips. Sprain is a self-limited benign process that resolves in 1-2 weeks. Symptomatic treatment consists of rest and oral analgesics.
The sacroiliac joint is particularly vulnerable to rotational injuries. It is one of the largest joints in the body and functions to transfer weight from the upper body to the lower extremities. Acute or chronic injury can cause slippage, or subluxation, of the joint. Pain originating from this joint is characteristically located along the posterior ilium and radiates down the hips and posterior thigh to the knees. The diagnosis is suggested by tenderness on palpation, particularly on the medial aspect of the posterior superior iliac spine, and by compression of the joints. Pain relief following injection of the joint with local anesthetic (3 mL) is diagnostic and may also be therapeutic. Injection of intraarticular steroid medication may be considered. For potentially longer duration of analgesia, radiofrequency ablation may be performed at the dorsal ramus of L5 as well as the lateral branches of the S1, S2, and S3 nerves if the patient responded well to local anesthetic injections of the sacroiliac joint or to diagnostic injections of these nerves.
Buttock pain may be due to several different factors, and can be quite debilitating. Coccydynia (or, coccygodynia) may the result of trauma to the coccyx or surrounding ligaments. It may resolve by means of physical therapy, coccygeal nerve blocks to the lateral aspects of the coccyx, or ablative or neuromodulatory techniques. Piriformis syndrome presents as pain in the buttock, which can be accompanied by numbness and tingling in distribution of the sciatic nerve. The nerve may or may not be entrapped. Injection of local anesthetic into the belly of this muscle or into trigger points located at the origin and insertion of the muscle may help relieve the pain.