Chronic Obstructive Pulmonary Disease
Albert Heurich MD
Chronic obstructive pulmonary disease (COPD) encompasses a number of disorders with the common feature of progressive and irreversible chronic airflow limitation. The two major disease states that make up COPD are chronic bronchitis and emphysema.
Bronchial asthma can be classified as a component of COPD because it often accompanies chronic bronchitis and emphysema. However, the obstructive component in asthma is usually acute and reversible. Asthma is discussed here only in its relation to COPD; further discussion of asthma is found in Chapter 72.
COPD has a long course of slow but progressive disability, with long periods of relatively stable health. The primary care provider can play a key role in maintaining baseline health for as long as possible and in helping the patient cope with the multiple issues this debilitating illness raises.
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
The airway changes in COPD that lead to obstruction include marked enlargement of bronchial mucous glands, gland duct dilation, hypertrophy of airway smooth muscle, and squamous metaplasia of the airway epithelium, causing hypersecretion of mucus and inflammation. The respiratory bronchioles show epithelial hyperplasia, increased numbers of pigmented macrophages, edema, and fibrosis. The membranous bronchioles are obstructed by inflammation, mucus plugs, goblet cell metaplasia, fibrosis, and increased smooth muscle.
The pathology of COPD is evident throughout the lungs, although the disease begins in the small airways. In the large airways there is enlargement of bronchial mucous glands and dilation of gland ducts. The surface lining can show focal squamous metaplasia, and there is an increased frequency of goblet cells. Bronchial smooth muscle shows hypertrophic changes.
Within respiratory bronchioles, inflammation is demonstrated by an increase of mononuclear cells. Additional changes include mucus plugging, increases in smooth muscle, goblet cell metaplasia, and fibrosis. The latter can distort the airway, leading to a further reduction in airflow.
Three forms of emphysema can be found in COPD:
Centriacinar emphysema initially involves the respiratory bronchioles, with later involvement of the acinus.
Centrilobular emphysema is associated with cigarette smoking and predominantly involves the upper lobes.
Panacinar emphysema uniformly affects the acinus and lower lobes.
Paraseptal emphysema occurs in adjacent areas to fibrous septa or pleura. Airflow limitation is mild, but it is implicated as a potential cause of pneumothorax.
Chronic respiratory acidosis, a common sequela to airway obstruction in COPD, is compensated by metabolic alkalosis. Alkalosis reduced respiratory center sensitivity to pCO2, which normally is the most potent respiratory center stimulus, leaving hypoxemia as the sole chemical stimulus to ventilation in COPD. This creates the conflicting situation of needing to administer oxygen to correct life-threatening hypoxemia and simultaneously removing the remaining stimulus to respiration. However, correction of alveolar hypoxia with oxygen relieves hypoxic pulmonary vasoconstriction in poorly ventilated regions of the lung. This increases pulmonary blood flow to the poorly ventilated areas, causing greater equilibration with the higher alveolar PCO2. Elimination of the hypoxemic stimulus to ventilation and the worsening of pulmonary ventilation/perfusion ratios from oxygen administration result in further hypercapnia. Minimizing the increase in PACO2 is accomplished by titration of the oxygen. The use of a device that can deliver high flows of precisely regulated oxygen concentrations usually permits the safe administration of oxygen in this setting.
Respiratory muscles respond to respiratory center drive with a pumping force that produces pulmonary gas exchange. Vagal afferent neural signals transmit information regarding pulmonary mechanics and lung volume, which the respiratory center uses to regulate its output to the muscles of respiration.
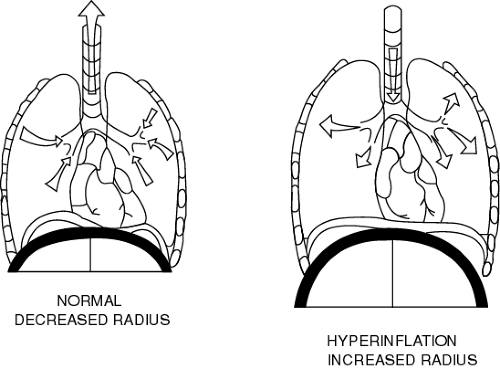
Functional residual capacity (FRC) reflects the balance between expiratory lung elastic recoil and inspiratory chest wall elastic recoil. Alveolar pressure is normally zero at FRC. Emphysema reduces lung elastic recoil, causing a new equilibrium of lung and chest wall elastic forces at an increased FRC.
Hyperinflation flattens the contour of the diaphragm and increases its radius of curvature (picture the diaphragm as part of the circumference of a circle that extends over the abdomen). It also shifts the operational lung compliance from the normal region to the low-compliance region, thus increasing pressure requirements for a tidal volume. The increased radius of curvature impairs diaphragmatic contractility and increases respiratory muscle work (Fig. 74-1).
Increased airway resistance prolongs expiration and produces premature airway closure. Premature airway closure results in the trapping of gas volume with positive alveolar pressures at end expiration (PEEP), also called auto-PEEP. This positive pressure must be overcome before inspiration can proceed. This increases the inspiratory work of breathing.
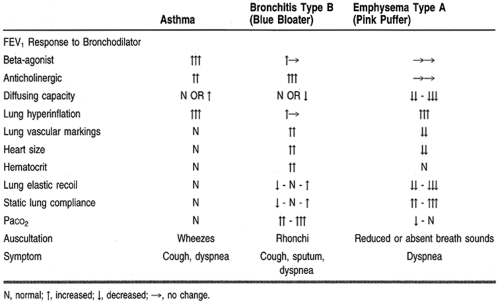
The emphysematous lung is ventilated but not perfused. It forms dead space and areas of low gas diffusion, increasing the work of breathing, although the resting blood gas analysis does not demonstrate this. The patient huffs and puffs to maintain ventilation and has thus been called a “pink puffer.”
In chronic obstructive bronchitis, poorly ventilated alveoli remain perfused. This causes hypoxemia with cyanosis and hypercapnia. Chronically elevated pulmonary vascular resistance
from hypoxia results in cor pulmonale. This is manifested by tricuspid valve insufficiency, right atrial dilatation, dilated neck veins, hepatic congestion, and pedal edema. The appearance of cyanosis with edema yields the designation “blue bloater” for such patients.
from hypoxia results in cor pulmonale. This is manifested by tricuspid valve insufficiency, right atrial dilatation, dilated neck veins, hepatic congestion, and pedal edema. The appearance of cyanosis with edema yields the designation “blue bloater” for such patients.
Total ventilation in these patients is high compared to that of normal persons, however. This reflects a high dead space ventilation and an unusually high respiratory drive. Emphysematous patients have a still higher respiratory drive.
The features of asthma, bronchitis, and emphysema are compared in Table 74-1.
EPIDEMIOLOGY
COPD affects 14.6 million Americans. Of these, approximately 12.6 million suffer from chronic bronchitis and 2 million have emphysema. These disorders caused 85,544 deaths in 1991. COPD ranks as the fourth leading cause of death, with a death rate of 18.6 per 100,000 persons. Since 1970, deaths related to COPD have doubled (Celli, 1998).
An increasing number of women are now dying from this disease; the male/female ratio has decreased from 4.3:1 in 1970 to 2.36:1 in 1983. The risk of death from COPD in heavy smokers is 30 times that for nonsmokers.
Alpha-1-antitrypsin (AAT) deficiency plays a relatively small role in the incidence of emphysema in the United States, accounting for less than 1%. The genetic locus responsible for AAT production is termed Pi. The M allele, and phenotype Pi MM, is found in 90% to 95% of the normal white population. Persons homozygous for the phenotype of the Z allele (Pi ZZ) manifest AAT deficiency. Those with disease have AAT levels that average 16% of normal.
DIAGNOSTIC CRITERIA
The diagnosis of COPD is made by clinical symptoms in chronic bronchitis, by anatomic changes in emphysema, and by functional impairment in asthma. The diagnosis of chronic bronchitis is based on the presence of a chronic cough with sputum production for 3 months per year in 2 successive years. The diagnosis
cannot be made if the patient has another disease explaining these symptoms. Chronic bronchitis accompanied by airway obstruction is called chronic obstructive bronchitis to distinguish it from simple bronchitis, which does not limit airflow.
cannot be made if the patient has another disease explaining these symptoms. Chronic bronchitis accompanied by airway obstruction is called chronic obstructive bronchitis to distinguish it from simple bronchitis, which does not limit airflow.
The diagnosis of emphysema is made by histologic findings of abnormally widened air spaces and destruction of lung tissue distal to the terminal bronchioles. Clinically the diagnosis is made by a combination of history, physical exam, and radiologic and pulmonary function findings. Alveolar destruction transforms the gas-exchanging surface into a nonfunctional air space. This is identified on pulmonary function testing as a decrease in the diffusing capacity of the lung.
The typical patient with COPD presents with overlapping features of more than one of these diseases. Most have a history of cigarette smoking, often exceeding 20 pack-years. However, only 15% of cigarette smokers develop clinically significant COPD. For patients who progress to disease, the long interval between the onset of smoking and the onset of clinical findings reflects the tremendous reserve of lung function that has to be destroyed before a patient becomes symptomatic. This also explains why most patients begin to seek help in their 50s and 60s.
Although most patients with COPD are older, long-time cigarette smokers, an important exception is the young patient with AAT deficiency emphysema. This inherited defect, characterized by a very early onset of emphysema, involves the lower lobes of the lung instead of the upper lobe, found in older patients. Smoking causes an even earlier onset of the disease. This defect can now be corrected with replacement of the deficient AAT, making an accurate diagnosis essential.
HISTORY AND PHYSICAL EXAM
Chronic cough with morning sputum production is the first symptom of chronic bronchitis. Sputum usually has a clear, mucoid appearance but becomes mucopurulent and thick and increases in quantity and frequency during acute exacerbations.
Lung hyperinflation may manifest as an increased anteroposterior diameter of the chest. In the stable patient, auscultation of the lung reveals decreased or absent sounds in emphysema and low-pitched sonorous rhonchi in bronchitis. During exacerbations, higher-pitched wheezes are heard. Dyspnea and tachypnea become more prominent and can progress to both inspiratory and expiratory use of accessory muscles of respiration. Increased intrapleural pressure excursions manifest as inspiratory intercostal muscle retractions, expiratory supraclavicular apical bulging, neck vein distention, and increased tracheal movement.
In severe exacerbations, prominent flaring of the alae nasi occurs. Patients place their elbows on firm surfaces, enabling the shoulder girdles to project away from the chest to enhance the effect of respiratory muscle motion.
During exacerbations of emphysema, patients become tachypneic and may manifest cyanosis if there is significant impairment of alveolar gas exchange. In the chronically hypoxemic patient, signs of cor pulmonale may be present. These include jugular venous distention, hepatomegaly, and pedal edema. Acute and chronic hypercapnia contribute to alveolar hypoxia and produce symptoms of headache and altered mental status.
Hypersomnolence may be an additional complication in the subgroup of COPD patients who have obstructive sleep apnea. This group is also polycythemic and appears plethoric. Patients with AAT deficiency typically present with complaints of cough, sputum production, and shortness of breath.
DIAGNOSTIC STUDIES
Radiologic tests are the chief diagnostic modality used in COPD. Computed tomography (CT) is a very sensitive tool in the detection of COPD and has facilitated early diagnosis, thus enabling intervention early in the disease.
Spirometry has also been used to detect COPD in its earliest stages. A commonly used test is the forced expiratory flow, which measures expiratory flow rates between 25% and 75% of the forced vital capacity (FVC), divided by the time between these two points. An isolated decrease in this flow rate may be an early indicator of COPD. At this stage, the abnormality is reversible with bronchodilator therapy.
Spirometric measurement of the ratio of the volume expired in 1 second (FEV1) divided by the maximal volume that can be exhaled from the lung after a maximal inspiration (FVC) gives a measure of the expiration rate, or the FEV1/FVC ratio. Airway obstruction is associated with a low FEV1/FVC ratio (Figs. 74-2, 74-3, and 74-4). This is related to a reduction in the FEV1. The response of the FEV1 to the administration of a bronchodilator establishes the degree of reversibility of the obstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree