TOPICS
2. Pertinent Physiologic Changes of Pregnancy
4. Resuscitation of the Pregnant Patient in Cardiopulmonary Arrest
INTRODUCTION
Pregnancy poses anatomic and physiologic impediments to cardiopulmonary resuscitation. Recent studies have shown that nearly 40% of obstetric providers are unaware of the pronounced differences between the resuscitation of pregnant women versus nonpregnant women.1 This chapter will review physiologic changes that occur in pregnancy, as well as causes of maternal arrest, which will form a foundation for understanding the modifications that must take place when resuscitating pregnant women.
PERTINENT PHYSIOLOGIC CHANGES OF PREGNANCY
The cardiovascular system changes dramatically throughout pregnancy. By 32 weeks’ gestation, cardiac output has increased 30% to 50% above baseline because of an increase in circulating blood volume (increased preload). Progesterone-induced smooth muscle relaxation decreases systemic vascular resistance, leading to reduced afterload and up to a 15% to 20% increase in heart rate. During labor, cardiac output will increase an additional 10% to 15% above baseline.2 It is imperative to understand that uterine blood flow is at its maximum by late pregnancy and cannot be further increased during hypoxic or low-flow states; thus, during cardiopulmonary arrest, there will be vasoconstriction of the uteroplacental bed, further compromising the fetus.3
A dilutional anemia occurs secondary to a 50% increase in plasma volume and only a 30% increase in red blood cell mass. Because of the increase in plasma volume, a substantial amount of hemorrhage can take place before signs of hypovolemia become apparent.1 This physiologic anemia may have an impact on oxygen delivery to vital organ systems, especially during cardiopulmonary arrest.3
By 20 weeks’ gestation, the uterus reaches the level of the inferior vena cava. With a pregnant woman in the supine position, the uterus may cause compression of the inferior vena cava and decrease cardiac output.2 Quantitative cardiovascular magnetic resonance imaging has shown a 24% decrease in cardiac output at 32 weeks’ gestation in the supine position as compared to the lateral position.4 By the third trimester, the uterus may completely obstruct the inferior vena cava, leading to syncope, hypotension, and bradycardia.5,6 Compression of pelvic veins is also a concern in the pregnant patient. Signs and symptoms of compression in the pelvis are dependent edema, venous stasis, varicose veins, and hemorrhoids.5 This increased venous pressure may lead to rapid blood loss in cases of injuries to the pelvis and lower extremities; therefore, it is best to avoid intravenous lines in lower extremities, especially for resuscitation efforts, because venous return from the lower extremities to the central circulation is not assured.1,5 Most importantly, in addition to compression of major vessels, the gravid uterus exerts pressure on the diaphragm, which may limit forward blood flow during chest compressions (Figure 30-1).3
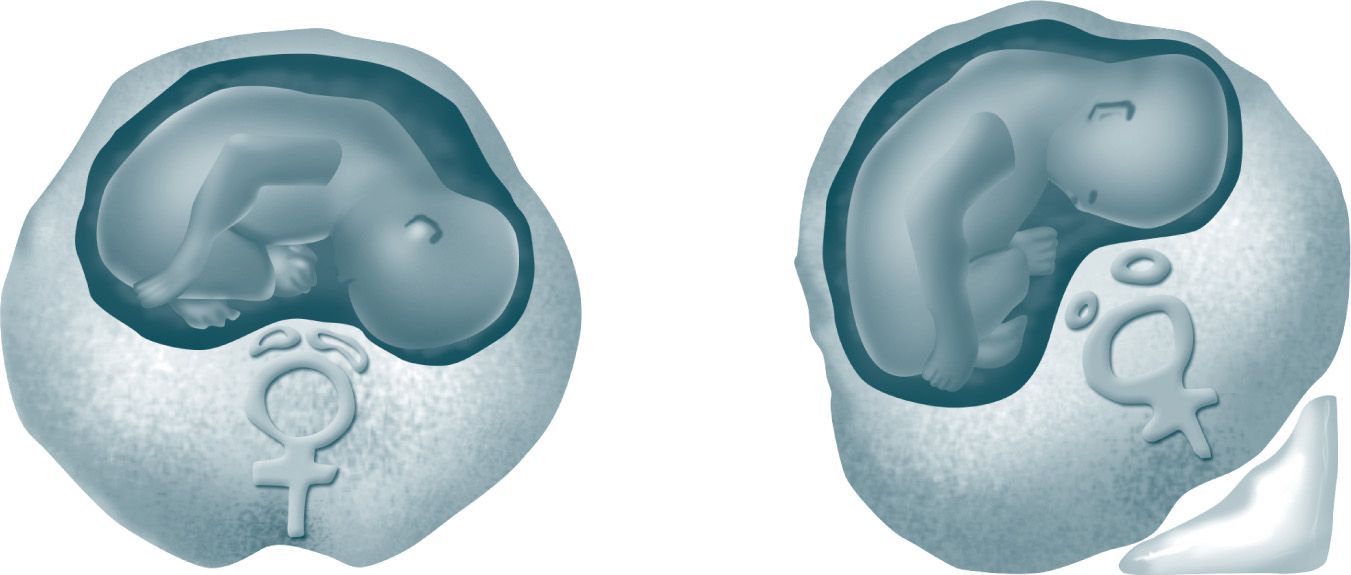
Figure 30-1. Aortocaval compression in the supine position and its alleviation with uterine displacement.
The gravid uterus affects not only the cardiovascular system but the respiratory system as well. As stated previously, the diaphragm will be displaced cephalad as a result of the gravid uterus.1,5 This leads to a 10% to 25% reduction in residual volume and functional residual capacity.2,3,5 Concurrently, maternal oxygen requirements increase 15% to 20%.1,5 The decrease in function residual capacity along with an increase in maternal oxygen demand causes hypoxia to occur quickly with respiratory arrest.2,3 The chronic respiratory alkalosis in pregnancy leads to a decrease in bicarbonate and thus a loss of blood-buffering capacity. The fetus is able to adapt to the chronic alkalosis by adjusting its carbon dioxide level and diffusing carbon dioxide from its higher fetal concentration to the lower maternal carbon dioxide concentration. Acute changes are more difficult for the fetus; when maternal hypercapnia occurs, as during cardiopulmonary arrest, the pressure gradient is altered, and the fetus cannot transfer as much carbon dioxide to the mother.7 Fortunately, the fetus is protected from asphyxia by several mechanisms. First, fetal hemoglobin carries 20% to 50% more oxygen than maternal hemoglobin, leading to a leftward shift of the fetal oxyhemoglobin dissociation curve. Second, fetal acidosis relative to the mother enhances oxygen uptake by fetal blood in the placental bed (Bohr effect). Third, fetal circulation during hypoxia causes increased blood flow to the fetal brain, heart, and adrenal glands. Theoretically, the fetus can survive hypoxia for greater than 10 minutes, although reports of intact fetal neurologic status and recovery from maternal arrest beyond 10 minutes are rare.8
The pregnant woman is at increased risk of aspiration secondary to a decrease in gastric motility and reduced gastric sphincter response.3,5 In addition, there is increased gastric acid production, which will theoretically increase pulmonary damage if aspiration occurs.5 These physiologic changes will mandate rapid airway management during cardiopulmonary resuscitation (CPR). Upper gastrointestinal changes that occur include edema and fragility of the pharynx and larynx secondary to an increase in estrogen and subsequent increase in interstitial water. A narrower airway lumen may be encountered secondary to pharyngeal and vocal cord edema, hindering the passage of the endotracheal tube; therefore, the use of a smaller than usual endotracheal tube may be prudent. A pregnant woman may have an increase in nasal mucosal congestion, especially after high blocks and/or labor; therefore, care should be taken when placing a nasogastric or nasotracheal tube, and these tubes should only be used when absolutely necessary.1
Pregnancy leads to an increase in volume of distribution. As a result, resuscitation drugs may be less effective, although dose adjustments are not generally recommended during initial resuscitative efforts. Although they are necessary for resuscitation, endogenous and exogenous catecholamines can vasoconstrict the uterine artery; this leads to a decrease in fetal oxygenation and fetal carbon dioxide exchange during resuscitation.3
CAUSES OF MATERNAL ARREST
Cardiopulmonary arrest in pregnant women is rare (incidence: 1 in 30,000 pregnancies).1 Having a thorough understanding of the physiologic changes that occur in pregnancy will help in determining the underlying cause of maternal arrest. This is important for improving maternal and fetal outcomes after cardiopulmonary arrest. The causes of arrest may be obstetric or nonobstetric (Table 30-1).
Table 30-1. Causes of Maternal Cardiac Arrest
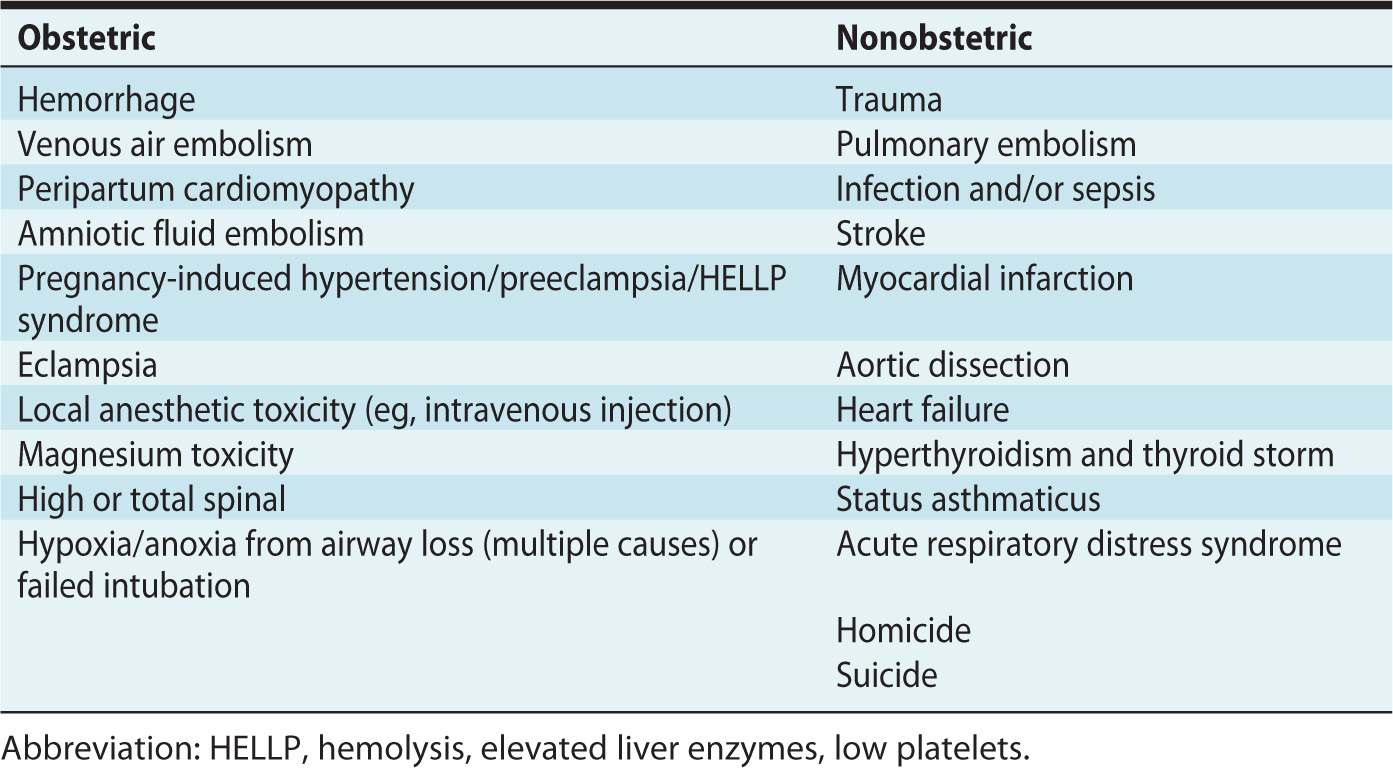
Hemorrhage is responsible for 25% of maternal deaths in pregnancy.5 Peripartum hemorrhage is frequently seen in cases of abruptio placentae, placenta previa, uterine rupture, and uterine atony, with atony being the most common cause of maternal hemorrhage. Massive hemorrhage and subsequent blood transfusion can result in acute respiratory distress syndrome (ARDS), which itself is associated with cardiac arrest and maternal death.3
Preeclampsia and the complications of preeclampsia (pulmonary edema, cardiac dysfunction, stroke, cerebral edema, and the hemolysis, elevated liver enzymes, low platelets [HELLP] syndrome) have also been implicated in cases of cardiac arrest.3 Although hypertension is observed in preeclampsia, these patients are often severely intravascularly volume depleted with significant third spacing of fluid, both of which increase the degree of difficulty in resuscitative efforts (including venous access, intubation, and response to hemodynamic support).
Abrupt cardiovascular collapse and coagulopathy during labor or immediately postpartum may be related to amniotic fluid embolism.5,9 Unfortunately, the mortality rate can be as high as 50%.3 This syndrome, sometimes called the “anaphylactoid syndrome of pregnancy,” may require the use of cardiopulmonary bypass. Bypass has been efficacious in managing cardiovascular collapse from severe pulmonary vasoconstriction.10,11
Anesthetic complications include anoxia and hypoxia from difficult intubation or aspiration, hemodynamic and respiratory collapse from a high or total spinal, and drug toxicity.12 Local anesthetic toxicity is an important concern in the pregnant woman. The American Society of Regional Anesthesia and Pain Medicine calls for specific guidelines, including immediate airway management, lower than normal doses of epinephrine (less than 1 μg/kg), avoidance of vasopressin, use of lipid emulsion, and notification of facilities having cardiopulmonary bypass capability when epinephrine and other therapies have failed.13
Idiopathic peripartum cardiomyopathy is rare. Typically seen during the last month of pregnancy through 6 months after delivery, it is cardiac failure in the absence of preexisting cardiac disease.2,3 Because symptoms of peripartum cardiomyopathy mirror those of pregnancy itself, the diagnosis is often overlooked, with cardiac arrest or sudden death being unfortunate presentations.
The nonobstetric causes of cardiopulmonary arrest are also numerous, as listed in Table 30-1. The pregnant patient presenting as a trauma warrants a careful examination of the abdomen, because the uterus displaces intra-abdominal organs and makes localization of injuries challenging. Due to desensitization of the abdominal wall, masking of abdominal pain and tenderness may occur. The patient should be assessed for the presence or absence of uterine contractions, uterine tenderness, or vaginal bleeding.5
The number one cause of nonobstetric maternal arrest is pulmonary thromboembolism. Thromboembolism occurs secondary to stasis and pregnancy-induced hypercoagulability. During uncomplicated pregnancies, women are hypercoagulable secondary to a net increase in clotting factors and prolonged bed rest during labor. Coexisting conditions, including factor V Leiden, antiphospholipid syndrome, and lupus erythematosus also contribute to hypercoagulability. Venous stasis, hypercoagulability, and vascular damage (Virchow triad) combine to make thromboembolism the leading cause of maternal death in developed countries.3,9
Pregnant women are particularly susceptible to infections such as chorioamnionitis, pneumonia, and urinary tract infections.3 In fact, urinary tract infections are the most common site of infection for critically ill obstetric patients.9 Septic shock, requiring fluid resuscitation and inotropic support, may occur as frequently as 1 in 5000 gestations.3
As women are waiting later in life to have children, the incidence of myocardial infarction during pregnancy has increased. The demands that pregnancy place on the cardiovascular system are great; this affects patients with preexisting cardiac disease.5 Risk factors for myocardial infarction during pregnancy include hypercholesterolemia, hypertension, diabetes mellitus, left ventricular hypertrophy, and smoking.3 Pregnant women are also at increased risk for spontaneous aortic dissection during pregnancy.5,10 This occurs because of hormonal changes on smooth muscle and connective tissue.5 Preexisting heart failure will affect management of the pregnant woman. Those with New York Heart Association class III or IV may require a pulmonary artery catheter to assess intravascular volume, cardiac output, and oxygen delivery.3
Poor control of asthma has been related to poor maternal and fetal outcomes, including preeclampsia, uterine hemorrhage, preterm delivery, and low birth weight.9 Other respiratory complications such as ARDS can occur secondary to pneumonia, sepsis, and/or amniotic fluid embolism.9
Pregnant women are often victims of domestic violence, making it imperative to consider attempted homicide and suicide when presented with an unexplained cardiopulmonary arrest. These are significant causes of mortality during pregnancy.10
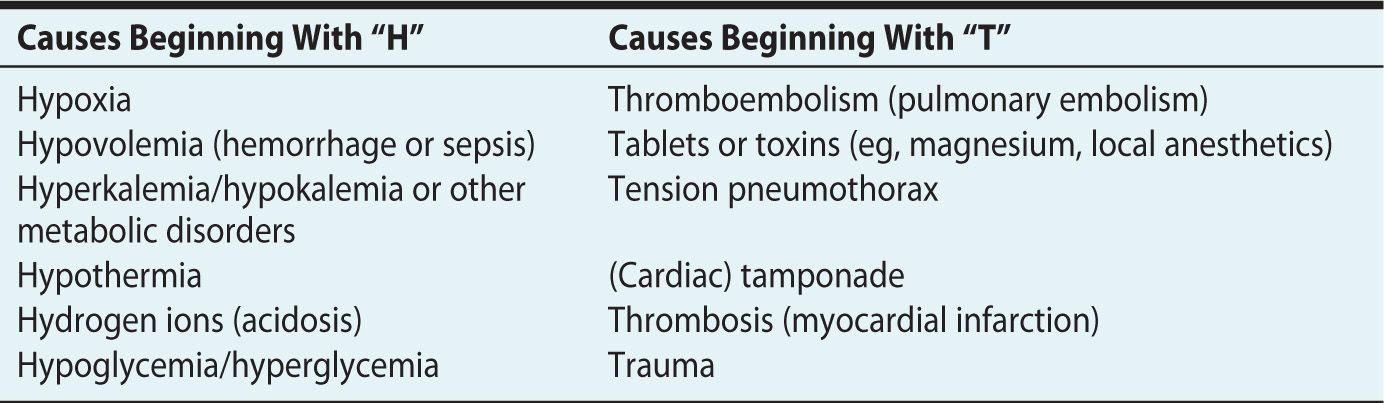
As with any patient presenting in cardiopulmonary arrest, it is important to remember reversible causes of cardiac arrest (Table 30-2).10,14
Table 30-2. Possible Causes of Cardiac Arrest
RESUSCITATION OF THE PREGNANT PATIENT IN CARDIOPULMONARY ARREST
Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) will not be reviewed here. Rather, the following is a guide to the modifications that must occur specific for the resuscitation of a pregnant woman to be successful. The treatment goal is to maintain adequate blood flow to optimize both maternal and fetal oxygenation (Table 30-3).15
Table 30-3. Modifications for Resuscitation of Pregnant Patients









