SCOPE OF THE PROBLEM
The actual incidence of neurologic dysfunction resulting from hemorrhagic complications associated with central neural blockade is unknown. In an extensive review of the literature, Tryba4 identified 13 cases of spinal hematoma following 850,000 epidural anesthetics and 7 cases among 650,000 spinal techniques. Based on these observations, the calculated incidence was approximated to be <1 in 150,000 epidural and <1 in 220,000 spinal anesthetics. Since these estimates represented the upper limit of the 95% confidence interval (CI), the overall frequency would presumably be much less. However, this estimation was performed in 1994, before the implementation of routine perioperative thromboprophylaxis. A more recent survey involving 1,710,000 neuraxial epidural blocks performed in Sweden over a 10-year period between 1990 and 1999 reported 33 spinal hematomas, for an overall frequency of 1.9 per 100,000 neuraxial anesthetics (95% CI 1.3–2.7 per 100,000).5 This epidemiologic study as well as other case series suggests a substantial increase in the frequency over the last two decades.5–7 Patient characteristics and anesthetic variables also modify the risk of spinal bleeding8 (Table 4-1). Although it is useful to identify patient populations at risk, even more crucial are management techniques that facilitate the detection and evaluation of new perioperative neurologic deficits, since neurologic outcome is dependent on timely intervention. Finally, improved perioperative outcomes associated with peripheral/plexus blocks have lead to an increased popularity of these techniques. However, it is important to note that serious hemorrhagic complications have also occurred with peripheral and plexus blockade.9–11
TABLE 4-1 Risk Factors and Estimated Incidence for Spinal Hematoma Associated with Neuraxial Anesthesia
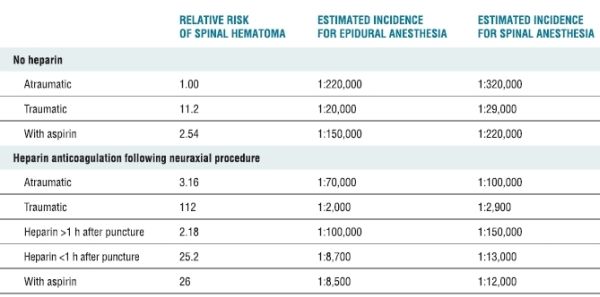
Data from Stafford-Smith M. Impaired haemostasis and regional anaesthesia. Can J Anaesth 1996;43:R129–R141. With permission.
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
The majority of spinal hematomas occur in the epidural space because of the prominent venous plexus.12 However, the actual source of the bleeding (arterial vs. venous) is controversial. Bleeding from an arterial source should accumulate rapidly and cause neural ischemia soon after vessel trauma. However, most spinal hematomas become symptomatic several days after needle/catheter placement, not immediately postoperatively, suggesting the bleeding is not arterial. On the other hand, a venous source would accumulate more slowly but theoretically would tamponade prior to overcoming spinal cord perfusion pressure. Thus, neither model entirely represents the clinical scenario. The volume of blood required to cause cord ischemia also varies and is affected by the site of bleeding (the cauda equina is relatively resistant, while the watershed areas of the cord are more easily compromised), the presence of vertebral column abnormalities, and the rapidity with which the blood accumulates. It is interesting to note that several of the LMWH hematomas involved less blood than that typically injected during the performance of an epidural blood patch.13
Of special interest to the anesthesiologist are those spinal hematomas that have occurred spontaneously in the patient receiving antithrombotic or antiplatelet therapy.14 Risk factors include the intensity of the anticoagulant effect, increased age, female gender, history of gastrointestinal bleeding, concomitant aspirin use, and length of therapy.15 During warfarin therapy, an international normalized ratio (INR) of 2.0 to 3.0 is associated with a low risk of bleeding: <3% during a 3-month treatment period. Higher intensity regimens (INR > 4) are associated with a significantly greater risk of bleeding (7%). The incidence of hemorrhagic complications during therapeutic anticoagulation with standard heparin, as well as LMWH, is <3%. Thrombolytic therapy represents the greatest risk of bleeding between 6% and 30% of patients.15
 RISK FACTORS
RISK FACTORS
An understanding of the mechanisms of blood coagulation, the pharmacologic properties of the anticoagulant and antiplatelet medications, and also the clinical studies involving patients undergoing central neural blockade while receiving these medications is paramount in reducing the risk of spinal hematoma in patients undergoing neuraxial blockade (Table 4-2). In a review of the literature between 1906 and 1994, Vandermeulen et al.12 reported 61 cases of spinal hematoma associated with epidural or spinal anesthesia. Included were five parturients and four patients with anatomic abnormalities of the spine, such as spina bifida occulta, spinal ependymoma, and spinal angioma. A spinal anesthetic was performed in 15 cases, the remaining 46 received an epidural technique. Of the 32 patients who underwent continuous epidural block, the hematoma occurred immediately at the time of catheter removal, suggesting that coagulation status at the time of catheter removal as well as placement should be considered.
TABLE 4.2 Pharmacologic Activities of Anticoagulants, Antiplatelet Agents, and Thrombolytics
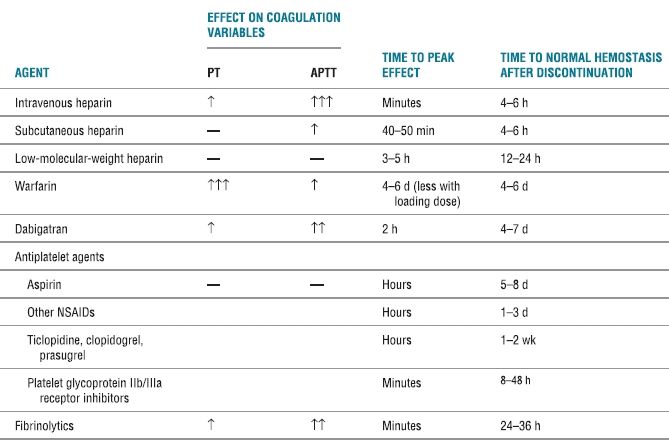
PT, prothrombin time; aPTT, activated partial thromboplastin time; ↑, clinically insignificant increase; ↑↑, possibly clinically significant increase; ↑↑↑, clinically significant increase; NSAID, nonsteroidal antiinflammatory drug.
In 42 of the 61 patients (68%), the spinal hematoma occurred in patients with evidence of hemostatic abnormality. Twenty-five patients had received intravenous heparin (18 patients), subcutaneous heparin (3 patients), or LMWH (4 patients), while an additional five patients presumably received heparin during a vascular surgical procedure. In addition, 12 patients had evidence of coagulopathy or thrombocytopenia or were treated with antiplatelet medications (aspirin, indomethacin, ticlopidine), oral anticoagulants (phenprocoumone), thrombolytics (urokinase), or dextran 70 immediately before or after the neuraxial anesthetic. Needle placement was reported as difficult in 25% of patients and/or bloody in 25% of patients. Multiple punctures were reported in 20% of patients. Therefore, in 87% of patients, a hemostatic abnormality or traumatic/difficult needle placement was present. More than one risk factor was present in 20 of 61 cases.
Neurologic compromise presented as progression of sensory or motor block (68% of patients) or bowel/bladder dysfunction (8% of patients), not as severe radicular back pain. Importantly, although only 38% of patients had partial or good neurologic recovery, spinal cord ischemia tended to be reversible in patients who underwent laminectomy within 8 hours of onset of neurologic dysfunction.
The need for prompt diagnosis and intervention in the event of a spinal hematoma was also demonstrated in a review of the American Society of Anesthesiologists (ASA) Closed Claims database which noted that spinal cord injuries were the leading cause of claims in the 1990s.16 Spinal hematomas accounted for nearly half of the spinal cord injuries. Risk factors for spinal hematoma included epidural anesthesia in the presence of intravenous heparin during a vascular surgical or diagnostic procedure. Importantly, the presence of postoperative numbness or weakness was typically attributed to local anesthetic effect rather than spinal cord ischemia, which delayed the diagnosis. Patient care was rarely judged to have met standards (1 of 13 cases) and the median payment was very high.
It is impossible to conclusively determine the risk factors for the development of spinal hematoma in patients undergoing neuraxial blockade solely through review of the case series, which represent only patients with the complication and do not define those who underwent uneventful neuraxial analgesia. However, large inclusive surveys which evaluate the frequencies of complications (including spinal hematoma), as well as identify subgroups of patients with higher or lower risk, enhance risk stratification. Moen et al.5 investigated serious neurologic complications among 1,260,000 spinal and 450,000 epidural blocks performed in Sweden over a 10-year period. Among the 33 spinal hematomas, 24 occurred in females; 25 were associated with an epidural technique. A coagulopathy (existing or acquired) was present in 11 patients; two of these patients were parturients with hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome. Spinal pathology was present in six patients. The presenting complaint was typically lower extremity weakness. Only 5 of the 33 patients recovered neurologically (due to delay in the diagnosis/intervention). These demographics, risk factors, and outcomes confirmed those of previous series. However, the methodology allowed for calculation of the frequency of spinal hematoma among patient populations. For example, the risk associated with epidural analgesia in women undergoing childbirth was significantly less (1 in 200,000) than that in elderly women undergoing knee arthroplasty (1 in 3,600, p < .0001). Likewise, women undergoing hip fracture surgery under spinal anesthesia had a statistically increased risk of spinal hematoma (1 in 22,000) compared to all patients undergoing spinal anesthesia (1 in 480,000).
Overall, these series report that the risk of clinically significant bleeding varies with age (and associated abnormalities of the spinal cord or vertebral column), the presence of an underlying coagulopathy, difficulty during needle placement, and an indwelling neuraxial catheter during sustained anticoagulation (particularly with standard heparin or LMWH), perhaps in a multifactorial manner. They also consistently demonstrate the need for prompt diagnosis and intervention.
Patient Risk Factors
Patient factors are not “controllable,” but should be considered when selecting a regional technique and the intensity of neurologic monitoring perioperatively. The patient factors for spinal hematoma are similar to those for spontaneous bleeding with antithrombotic therapy—increased age, female gender, and concomitant hepatic or renal disease (which exaggerate the anticoagulant response).5,13,15 In a series of 40 spinal hematomas associated with LMWH thromboprophylaxis, 75% of the patients were elderly women.13 Similarly, Moen et al. noted that 70% of patients with spinal hematoma were females and often had preexisting spinal stenosis due to osteoporosis.5 The increased frequency among elderly women may be due to increased sensitivity to thromboprophylactic medications and/or changes in the vertebral column with age.
Since pregnancy and the immediate postpartum interval are associated with a hypercoagulable state, it is often assumed that parturients are not at risk for spinal hematoma. However, in the series by Vandermeulen et al.12 5 of 61 spinal hematomas involved parturients. In two cases, a clotting disorder (thrombocytopenia, preeclampsia) was present. One parturient had a previously undiagnosed epidural ependymoma. No risk factors were reported in the remaining two patients. The two parturients in the series by Moen et al.5 occurred in the presence of severe coagulopathy. To date, there have been no published spinal hematomas associated with peripartum antithrombotic therapy. However, there is no large series documenting the safety of neuraxial block in the presence of the therapeutic levels of anticoagulation required among this patient population. Therefore, the relative risk is unknown.
Anesthetic Risk Factors
Anesthetic variables that may affect the risk of spinal hematoma include needle/catheter gauge, the trauma incurred during needle/catheter insertion, and the placement of an indwelling neuraxial catheter. Although the presence of blood during neuraxial block does not portend spinal hematoma, traumatic needle or catheter placement has often been described in case reports of spinal hematoma.12,13,17,18 In addition, larger gauge needles and the insertion of an epidural or spinal catheter increase the likelihood of traumatic needle placement,17,19 and nearly three-fourths of spinal hematomas are associated with a continuous catheter technique.5,12,13 Several studies have verified that the placement of an indwelling intrathecal or epidural catheter increased the risk of minor (clinically insignificant) spinal bleeding.17,19 Horlocker et al.17 reported that blood was present during the placement of 18 of 46 (39%) intrathecal or 138 of 575 (24%) epidural catheters. This was significantly higher than the frequency associated with single-dose spinal anesthesia, 64 of 362 (18%). Patients with blood present during needle and catheter placement were more likely to have blood present in the catheter at the time of removal. These investigations demonstrate that indwelling neuraxial catheters result in trauma to spinal vasculature. The presence of anticoagulation theoretically increases the chance that bleeding will be increased and may become clinically significant, particularly when the catheter is removed during altered hemostasis.12,16
Pharmacologic Actions and Interactions
Patients react with different sensitivities to anticoagulants. Highly sensitive patients will exhibit a greater increase in the degree of anticoagulation and prolonged effect after discontinuation of the medication. A single dose (3–5 mg) of warfarin resulted in the prolongation of the prothrombin time (PT) in approximately 20% of patients.20 Conversely, in resistant patients, the anticoagulant effects will be decreased and short-lived. For example, patients with acute thromboembolic disease exhibit heparin resistance secondary to a reduction in anti-thrombin III. A number of factors affect a patient’s sensitivity to heparin and warfarin including overall medical condition, diet, renal function, and liver disease (Box 4-1). Finally, the anticoagulant effects may be potentiated by the administration of concomitant medications, such as nonsteroidal antiinflammatory medications or dextran. Therefore, it is imperative that the clinician be knowledgeable of the patient’s preexisting medical conditions, the anticipated method and the level of perioperative anticoagulation, and the potential for drug interactions. In addition, anticoagulant activity should be closely monitored, particularly in patients with indwelling catheters.
BOX 4-1 Summary of Patient Characteristics Associated with Enhanced Prothrombin Time Response to Warfarin
 Age >65 years
Age >65 years
 Female gender
Female gender
 Weight <100 lbs
Weight <100 lbs
 Excessive surgical blood loss
Excessive surgical blood loss
 Liver, cardiac, renal disease
Liver, cardiac, renal disease
 Oriental race
Oriental race
 CYP2C9 and/or VKORC1 genetic variation
CYP2C9 and/or VKORC1 genetic variation
Preoperative Anticoagulation
Patients who require chronic anticoagulant therapy, such as those with a history of atrial fibrillation or cardiac valve replacement, are not ideal candidates for neuraxial techniques. Often the anticoagulant effect is only partially reversed to avoid thrombotic complications and/or they are aggressively anticoagulated postoperatively. Normal hemostasis for needle/catheter placement is present only for a short interval and may not be maintained (making catheter removal problematic).
Unanticipated Anticoagulation or Thrombolysis
Patients who have recently undergone neuraxial anesthesia and require emergent anticoagulation for limb ischemia, acute coronary syndrome, or deep-venous thrombosis/pulmonary embolism represent a conundrum for the anesthesiologist. Ideally, the patient should be queried prior to antithrombotic or thrombolytic therapy for a recent history of lumbar puncture, spinal or epidural anesthesia, or epidural steroid injection to allow appropriate management and monitoring. The decision to maintain or remove an existing neuraxial catheter is based on the degree/duration of antithrombotic therapy. Since combination therapy (antiplatelet medications with heparin and/or thrombolytics) represents a greater risk of bleeding, treatment options with the least impact on coagulation should be considered.15 Unfortunately, while the anesthesia community is well aware of the potential for spinal bleeding in this patient population, other specialties have only recently become cognizant of the risk.21 As a result, the anesthesiologist may not be notified until after the establishment of therapeutic anticoagulation. Under these circumstances, a consensus on when hemostasis may be restored to allow catheter removal must be achieved, balancing the relative risks of hemorrhage and thrombosis. Complete reversal of the anticoagulant effect may not be feasible due to the risk of thromboembolic complications. Most importantly, ongoing efforts to educate clinicians responsible for administering hemostasis-altering medications are critical.
 GUIDELINES FOR ANTITHROMBOTIC THERAPY
GUIDELINES FOR ANTITHROMBOTIC THERAPY
In 2008, the American College of Chest Physicians (ACCP) released the proceedings of the Eighth Conference on Antithrombotic and Thrombolytic Therapy3 (Table 4-3). These recommendations represent new challenges in the management of patients undergoing neuraxial (and invasive/noncompressible peripheral) blockade. In general, higher degrees of thromboprophylaxis for extended intervals are recommended (Box 4-2). An acceptable alternative to the ACCP guidelines are those developed by the Surgical Care Improvement Project (SCIP, www.qualitynet.org). In addition, it is important to note that in response to ongoing concerns regarding surgical bleeding associated with thromboprophylaxis, the American Academy of Orthopaedic Surgeons (AAOS) also published guidelines in 2007 for the prevention of symptomatic pulmonary embolism (rather than deep-venous thrombosis) in patients undergoing total joint replacement (www.aaos.org/guidelines.pdf). In general, the AAOS guidelines are more conservative and recommend routine mechanical prophylaxis and aggressive chemoprophylaxis for higher risk patients only.
TABLE 4.3 Pharmacological Venous Thromboembolism Prophylaxis and Treatment Regimens
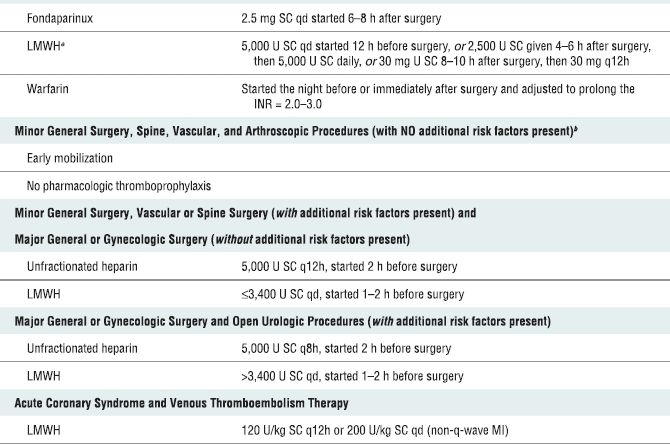
SC, subcutaneous; LMWH, low-molecular-weight heparin; INR, international normalized ratio.
aLMWH formulations available in North America are enoxaparin and dalteparin.
bThe risk factors for thromboembolism include trauma, immobility/paresis, malignancy, previous thromboembolism, increasing age (over 40 years), pregnancy, estrogen therapy, obesity, smoking history, varicose veins, and inherited or congenital thrombophilia. Based on recommendations from Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:381S–453S. With permission.
BOX 4-2 Trends in Thromboprophylaxis That May Increase the Risk of Spinal Hematoma
 Thromboprophylaxis (e.g., standard and LMWH for patients undergoing general surgery) is often administered in close proximity to surgery. Unfortunately, early postoperative dosing is associated with surgical (and often anesthesia-related) bleeding.
Thromboprophylaxis (e.g., standard and LMWH for patients undergoing general surgery) is often administered in close proximity to surgery. Unfortunately, early postoperative dosing is associated with surgical (and often anesthesia-related) bleeding.
 Fondaparinux is recommended as an anti-thrombotic agent following major orthopedic surgery. The extended half-life (~ 20 h) impedes safe catheter removal.
Fondaparinux is recommended as an anti-thrombotic agent following major orthopedic surgery. The extended half-life (~ 20 h) impedes safe catheter removal.
 LMWH and dabigatran are dependent on renal metabolism. Dose adjustment for decreased weight and/or renal function is not performed routinely and may result in an exaggerated and prolonged effect.
LMWH and dabigatran are dependent on renal metabolism. Dose adjustment for decreased weight and/or renal function is not performed routinely and may result in an exaggerated and prolonged effect.
 The duration of prophylaxis has been extended to include “postdismissal” administration. It has been demonstrated that the risk of bleeding complications is increased with the duration of anticoagulant therapy.
The duration of prophylaxis has been extended to include “postdismissal” administration. It has been demonstrated that the risk of bleeding complications is increased with the duration of anticoagulant therapy.
 Newly released and investigational antithrombotic agents are associated with prolonged half-lives, are not routinely monitored for anticoagulant effect, and do not have pharmacologic antidotes.
Newly released and investigational antithrombotic agents are associated with prolonged half-lives, are not routinely monitored for anticoagulant effect, and do not have pharmacologic antidotes.
Intravenous and Subcutaneous Standard Heparin
Complete systemic heparinization is usually reserved for the most high-risk patients, typically patients with an acute thromboembolism. However, intraoperative administration of a modest intravenous dose is occasionally performed during vascular or orthopedic procedures. In a study involving over 4,000 patients, Rao and El-Etr22 demonstrated the safety of indwelling spinal and epidural catheters during systemic heparinization. However, the heparin activity was closely monitored, the indwelling catheters were removed at a time when circulating heparin levels were relatively low, and patients with a preexisting coagulation disorder were excluded. A subsequent study in the neurologic literature by Ruff and Dougherty18 reported spinal hematomas in 7 of 342 patients (2%) who underwent a diagnostic lumbar puncture and subsequent heparinization. Traumatic needle placement, initiation of anticoagulation within 1 hour of lumbar puncture, or concomitant aspirin therapy were identified as risk factors in the development of spinal hematoma in anticoagulated patients (Table 4-1). Overall, large published series and extensive clinical experience suggests that the use of regional techniques during systemic heparinization does not appear to represent a significant risk. However, the cases of paralysis relating to spinal hematoma in the ASA Closed Claims database suggest that these events continue to occur and that extreme vigilance is necessary to diagnose and intervene as early as possible, should spinal hematoma be suspected.16
Since the publication of the initial American Society of Regional Anesthesia and Pain Medicine (ASRA) guidelines in 1998,23 there have been continued discussions regarding the relative risk (and benefit) of neuraxial anesthesia and analgesia in the patient undergoing heparinization for cardiopulmonary bypass. Further reports of small series have appeared, again with no reported complications. To date, there is a single case of spinal hematoma following the full heparinization associated with cardiopulmonary bypass.24 However, there are confounding variables in that the patient was initially neurologically intact, but developed paraplegia after anticoagulation/thrombolysis on the second day. Thus, this analgesic technique remains controversial in that the risk appears too great for the perceived benefits. A review has recommended certain precautions to be taken to minimize the risk25:
1. Neuraxial blocks should be avoided in a patient with known coagulopathy from any cause,
2. Surgery should be delayed 24 hours in the event of a traumatic tap,
3. Time from instrumentation to systemic heparinization should exceed 60 minutes,
4. Heparin effect and reversal should be tightly controlled (smallest amount of heparin for the shortest duration compatible with therapeutic objectives),
5. Epidural catheters should be removed when normal coagulation is restored, and patients should be closely monitored postoperatively for signs and symptoms of hematoma formation.
Furthermore, Ho et al. calculated the risk of hematoma using a complex mathematical analysis of the probability of predicting a rare event that has not occurred yet; they estimate the probability of a spinal hematoma (based on the totals of 4,583 epidural and 10,840 spinal anesthetics reported without complications) to be in the neighborhood of 1:1,528 for epidural and 1:3,610 for spinal technique.26 The theoretically increased risk of spinal hematoma and lack of substantial improvement in morbidity and mortality associated with neuraxial techniques among patients undergoing cardiopulmonary bypass warrants caution in this approach.27
Administration of 5,000 units of heparin subcutaneously every 12 hours has been used extensively and effectively for prophylaxis against deep-venous thrombosis. There is often no detectable change in the clotting parameters, as measured by the aPTT. There is a minority of patients, perhaps up to 15%, who may develop measurable changes in coagulation, although the aPTT rarely exceeds 1.5 times the normal level.28 There is a smaller subset (2%–4%) of patients who may become therapeutically anticoagulated during subcutaneous heparin therapy. With therapy >5 days, there is a subset of patients who will develop a decrease in the platelet count.29
The widespread use of subcutaneous heparin and paucity of complications suggests that there is little risk of spinal hematoma associated with this therapy. There are nine published series totaling over 9,000 patients who have received this therapy without complications,23 as well as extensive experience in both Europe and United States without a significant frequency of complications. There are only five case reports of neuraxial hematomas, four epidural,12,30 and one subarachnoid,31 during neuraxial block with the use of subcutaneous heparin.
The relative safety of higher doses (e.g., thrice daily dosing) of subcutaneous heparin is unknown. The clinician is currently faced with a decision to proceed with epidural analgesia, as there are no data of concern, or to take a more anticipatory approach of caution, awaiting adverse reports such as may appear in the ASA closed claims database. A review of relevant literature shows that there are reports that document an increased risk of minor and major bleeding in surgical and in nonsurgical patients receiving thrice daily subcutaneous UFH.32,33 Until more information is provided, a somewhat more cautious approach is suggested, such as enhanced neurologic monitoring and avoidance of additional hemostasis-altering drugs in these patients.
Low-Molecular-Weight Heparin
Prior to the introduction of LMWH for thromboprophylaxis following major orthopedic surgery, spinal hematoma was rarely reported. However, a total of 30 cases of spinal hematoma in patients undergoing spinal or epidural anesthesia while receiving LMWH perioperatively were reported between May 1993 and November 1997 through the MedWatch system.13 An FDA Health Advisory was issued in December 1997, and the manufacturers of all LMWH and heparinoids were requested to revise the labeling of their respective products and place a “black box warning.”
The majority of spinal hematomas were associated with an epidural technique. Early postoperative (or intraoperative) LMWH administration and concomitant antiplatelet therapy have been identified as risk factors for both increased surgical and anesthetic related bleeding complications5,6,13 (Box 4-3). The risk of spinal hematoma in patients receiving LMWH was estimated to be approximately 1 in 3,000 continuous epidural anesthetics compared to 1 in 40,000 spinal anesthetics.7. However, this was most likely an underestimation—there were a number of spinal hematomas that had occurred but had not been reported (and therefore included in the calculations). In total, nearly 60 spinal hematomas were tallied by the FDA between 1993 and 1998.34 This is noteworthy in that Vandermeulen et al.12 had identified a total of 61 spinal hematomas in the first 100 years of neuraxial blockade. Although it initially appeared that once daily dosing of LMWH allowed for safe maintenance of indwelling neuraxial catheters, new information from Sweden and Germany suggests that the risk may be nearly as high as that associated with twice daily dosing.5,35
BOX 4-3 Patient, Anesthetic, and Low-Molecular-Weight Heparin (LMWH) Dosing Variables Associated with Spinal Hematoma
Patient factors
Female gender
Increased age
Spinal stenosis or ankylosing spondylitis
Impaired renal function
Anesthetic factors
Traumatic needle/catheter placement
Epidural (compared to spinal) technique
Indwelling epidural catheter during LMWH administration
LMWH dosing factors
Immediate preoperative or intraoperative LMWH administration
Early postoperative administration
Twice daily dosing
Concomitant antiplatelet or anticoagulant medications
Adapted from Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med 2003;28:172–197. With permission.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


