Key Concepts
 During one-lung ventilation, the mixing of unoxygenated blood from the collapsed upper lung with oxygenated blood from the still-ventilated dependent lung widens the alveolar-to-arterial (A-a) O2 gradient and often results in hypoxemia.
During one-lung ventilation, the mixing of unoxygenated blood from the collapsed upper lung with oxygenated blood from the still-ventilated dependent lung widens the alveolar-to-arterial (A-a) O2 gradient and often results in hypoxemia.
 There are certain clinical situations in which the use of a right-sided double-lumen tube is recommended: (1) distorted anatomy of the left main bronchus by an intrabronchial or extrabronchial mass; (2) compression of the left main bronchus due to a descending thoracic aortic aneurysm; (3) left-sided pneumonectomy; (4) left-sided single lung transplantation; and (5) left-sided sleeve resection.
There are certain clinical situations in which the use of a right-sided double-lumen tube is recommended: (1) distorted anatomy of the left main bronchus by an intrabronchial or extrabronchial mass; (2) compression of the left main bronchus due to a descending thoracic aortic aneurysm; (3) left-sided pneumonectomy; (4) left-sided single lung transplantation; and (5) left-sided sleeve resection.
 If epidural opioids are to be used postoperatively, their intravenous use should be limited during surgery to prevent excessive postoperative respiratory depression.
If epidural opioids are to be used postoperatively, their intravenous use should be limited during surgery to prevent excessive postoperative respiratory depression.
 Postoperative hemorrhage complicates about 3% of thoracotomies and may be associated with up to 20% mortality. Signs of hemorrhage include increased chest tube drainage (>200 mL/h), hypotension, tachycardia, and a falling hematocrit.
Postoperative hemorrhage complicates about 3% of thoracotomies and may be associated with up to 20% mortality. Signs of hemorrhage include increased chest tube drainage (>200 mL/h), hypotension, tachycardia, and a falling hematocrit.
 Bronchopleural fistula presents as a sudden large air leak from the chest tube that may be associated with an increasing pneumothorax and partial lung collapse.
Bronchopleural fistula presents as a sudden large air leak from the chest tube that may be associated with an increasing pneumothorax and partial lung collapse.
 Acute herniation of the heart into the operative hemithorax can occur through the pericardial defect that is left following a radical pneumonectomy.
Acute herniation of the heart into the operative hemithorax can occur through the pericardial defect that is left following a radical pneumonectomy.
 Nitrous oxide is contraindicated in patients with cysts or bullae because it can expand the air space and cause rupture. The latter may be signaled by sudden hypotension, bronchospasm, or an abrupt rise in peak inflation pressure and requires immediate placement of a chest tube.
Nitrous oxide is contraindicated in patients with cysts or bullae because it can expand the air space and cause rupture. The latter may be signaled by sudden hypotension, bronchospasm, or an abrupt rise in peak inflation pressure and requires immediate placement of a chest tube.
 Following transplantation, peak inspiratory pressures should be maintained at the minimum pressure compatible with good lung expansion, and the inspired oxygen concentration should be maintained as close to room air as allowed by a Pao2 >60 mm Hg.
Following transplantation, peak inspiratory pressures should be maintained at the minimum pressure compatible with good lung expansion, and the inspired oxygen concentration should be maintained as close to room air as allowed by a Pao2 >60 mm Hg.
 Regardless of the procedure, a common anesthetic concern for patients with esophageal disease is the risk of pulmonary aspiration.
Regardless of the procedure, a common anesthetic concern for patients with esophageal disease is the risk of pulmonary aspiration.
Anesthesia for Thoracic Surgery: Introduction
Indications and techniques for thoracic surgery continually evolve. Common indications now include thoracic malignancies (mainly of the lungs and esophagus), chest trauma, esophageal disease, and mediastinal tumors. Diagnostic procedures such as bronchoscopy, mediastinoscopy, and open-lung biopsies are also common. Anesthetic techniques for providing lung separation have allowed the refinement of surgical techniques to the point that many procedures are increasingly performed thoracoscopically.
Physiological Considerations during Thoracic Anesthesia
Thoracic surgery presents a unique set of physiological problems for the anesthesiologist. These include physiological derangements caused by placing the patient in the lateral decubitus position, opening the chest (open pneumothorax), and the need for one-lung ventilation.
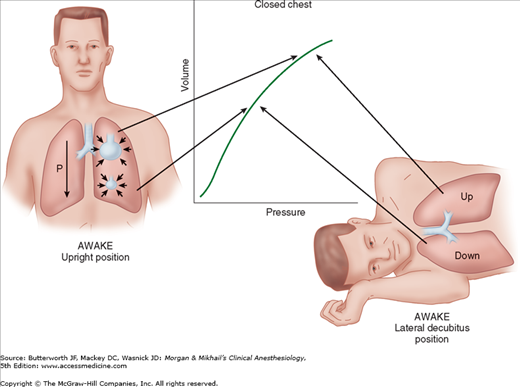
The lateral decubitus position provides optimal access for most operations on the lungs, pleura, esophagus, the great vessels, other mediastinal structures, and vertebrae. Unfortunately, this position may significantly alter the normal pulmonary ventilation/perfusion relationships. These derangements are further accentuated by induction of anesthesia, initiation of mechanical ventilation, neuromuscular blockade, opening the chest, and surgical retraction. Although perfusion continues to favor the dependent (lower) lung, ventilation progressively favors the less perfused upper lung. The resulting mismatch increases the risk of hypoxemia.
When a supine patient assumes the lateral decubitus position, ventilation/perfusion matching is preserved during spontaneous ventilation. The dependent (lower) lung receives more perfusion than does the upper lung due to gravitational influences on blood flow distribution in the pulmonary circulation. The dependent lung also receives more ventilation because: (1) contraction of the dependent hemidiaphragm is more efficient compared with the nondependent [upper] hemidiaphragm and (2) the dependent lung is on a more favorable part of the compliance curve (Figure 25-1).
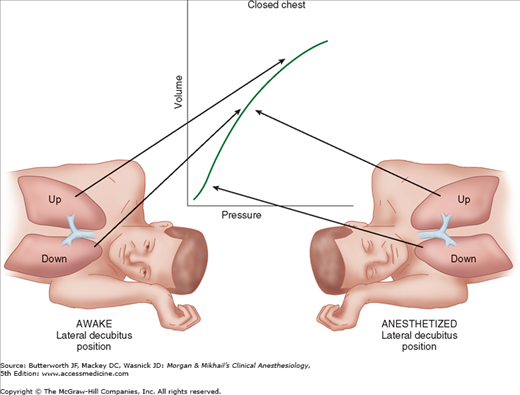
The decrease in functional residual capacity (FRC) with induction of general anesthesia moves the upper lung to a more favorable part of the compliance curve, but moves the lower lung to a less favorableposition (Figure 25-2). As a result, the upper lung is ventilated more than the dependent lower lung; ventilation/perfusion mismatching occurs because the dependent lung continues to have greater perfusion.
Controlled positive-pressure ventilation favors the upper lung in the lateral position because it is more compliant than the lower lung. Neuromuscular blockade enhances this effect by allowing the abdominal contents to rise up further against the dependent hemidiaphragm and impede ventilation of the lower lung. Using a rigid “bean bag” to maintain the patient in the lateral decubitus position further restricts movement of the dependent hemithorax. Finally, opening the nondependent side of the chest further accentuates differences in compliance between the two sides because the upper lung is now less restricted in movement. All of these effects worsen ventilation/perfusion mismatching and predispose the patient to hypoxemia.
The lungs are normally kept expanded by a negative pleural pressure—the net result of the tendency of the lung to collapse and the chest wall to expand. When one side of the chest is opened, the negative pleural pressure is lost, and the elastic recoil of the lung on that side tends to collapse it. Spontaneous ventilation with an open pneumothorax in the lateral position results in paradoxical respirations and mediastinal shift. These two phenomena can cause progressive hypoxemia and hypercapnia, but, fortunately, their effects are overcome by the use of positive-pressure ventilation during general anesthesia and thoracotomy.
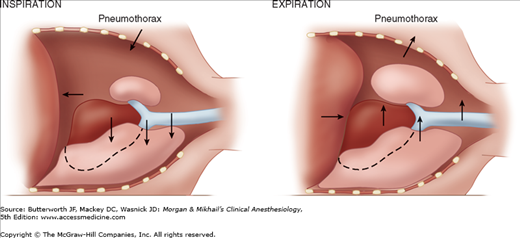
During spontaneous ventilation in the lateral position, inspiration causes pleural pressure to become more negative on the dependent side, but not on the side of the open pneumothorax. This results in a downward shift of the mediastinum during inspiration and an upward shift during expiration (Figure 25-3). The major effect of the mediastinal shift is to decrease the contribution of the dependent lung to the tidal volume.
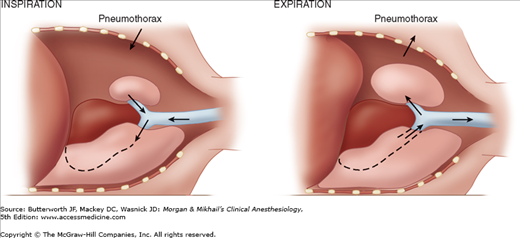
Spontaneous ventilation in a patient with an open pneumothorax also results in to-and-fro gas flow between the dependent and nondependent lung (paradoxical respiration [pendeluft]). During inspiration, the pneumothorax increases, and gas flows from the upper lung across the carina to the dependent lung. During expiration, the gas flow reverses and moves from the dependent to the upper lung (Figure 25-4).
Intentional collapse of the lung on the operative side facilitates most thoracic procedures, but greatly complicates anesthetic management. Because the collapsed lung continues to be perfused and is deliberately no longer ventilated, the patient develops a large right-to-left intrapulmonary shunt (20% to 30%).  During one-lung ventilation, the mixing of unoxygenated blood from the collapsed upper lung with oxygenated blood from the still-ventilated dependent lung widens the alveolar-to-arterial (A-a) O2 gradient and often results in hypoxemia. Fortunately, blood flow to the nonventilated lung is decreased by hypoxic pulmonary vasoconstriction (HPV) and possibly surgical compression of the upper lung.
During one-lung ventilation, the mixing of unoxygenated blood from the collapsed upper lung with oxygenated blood from the still-ventilated dependent lung widens the alveolar-to-arterial (A-a) O2 gradient and often results in hypoxemia. Fortunately, blood flow to the nonventilated lung is decreased by hypoxic pulmonary vasoconstriction (HPV) and possibly surgical compression of the upper lung.
Factors known to inhibit HPV (increasing venous admixture), and thus worsen the right-to-left shunting, include (1) very high or very low pulmonary artery pressures; (2) hypocapnia; (3) high or very low mixed venous Po2; (4) vasodilators such as nitroglycerin, nitroprusside, phosophodiesterase inhibitors (milrinone and inamrinone), β-adrenergic agonists, calcium channel blockers; (5) pulmonary infection; and (6) inhalation anesthetics.
Factors that decrease blood flow to the ventilated lung can be equally detrimental; they counteract the effect of HPV by indirectly increasing blood flow to the collapsed lung. Such factors include (1) high mean airway pressures in the ventilated lung due to high positive end-expiratory pressure (PEEP), hyperventilation, or high peak inspiratory pressures; (2) a low Fio2, which produces hypoxic pulmonary vasoconstriction in the ventilated lung; (3) vasoconstrictors that may have a greater effect on normoxic vessels than hypoxic ones; and (4) intrinsic PEEP that develops due to inadequate expiratory times.
Elimination of CO2 is usually unchanged by one-lung ventilation, provided that minute ventilation is unchanged and that preexisting CO2 retention was not present while ventilating both lungs; arterial CO2 tension is usually not appreciably altered.
Techniques for One-Lung Ventilation
One-lung ventilation can also be utilized to isolate a lung or to facilitate ventilatory management under certain conditions (Table 25-1). Three techniques can be employed: (1) placement of a double-lumen bronchial tube; (2) use of a single-lumen tracheal tube in conjunction with a bronchial blocker; or (3) insertion of a conventional endotracheal tube into a mainstem bronchus. Double-lumen tubes are most often used.
|
The principal advantages of double-lumen tubes are relative ease of placement, the ability to ventilate one or both lungs, and the ability to suction either lung.
All double-lumen tubes share the following characteristics:
- A longer bronchial lumen that enters either the right or left main bronchus and another shorter tracheal lumen that terminates in the lower trachea
- A preformed curve that when properly “aimed” allows preferential entry into abronchus
- A bronchial cuff
- A tracheal cuff
Ventilation can be delivered to only one lung by clamping either the bronchial or tracheal lumen with both cuffs inflated; opening the port on the appropriate connector allows the ipsilateral lung to collapse. Because of differences in bronchial anatomy between the two sides, tubes are designed specifically for either the right or left bronchus. A right-sided double-lumen tube incorporates a modified cuff and a proximal portal on the endobronchial side for ventilation of the right upper lobe. The most commonly used double-lumen tube are available in several sizes: 35, 37, 39, and 41F.
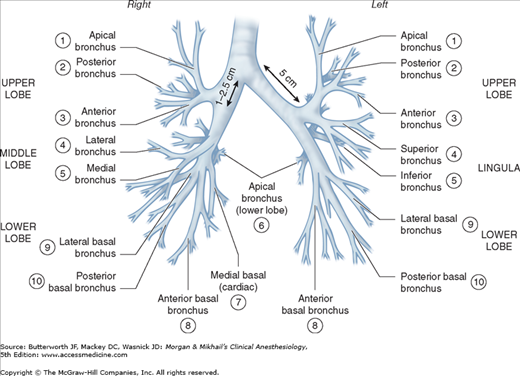
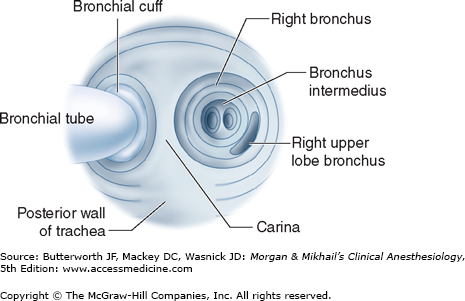
On average, the adult trachea is 11-13 cm long. It begins at the level of the cricoid cartilage (C6) and bifurcates at the level of the carina behind the sternomanubrial joint (T5). Major differences between the right and left main bronchi are as follows: (1) the larger diameter right bronchus diverges away from the trachea at a less acute angle in relation to the trachea, whereas the left bronchus diverges at a more horizontal angle (Figure 25-5); (2) the right bronchus has upper, middle, and lower lobe branches, whereas the left bronchus divides into only upper and lower lobe branches; and (3) the orifice of the right upper lobe bronchus is typically about 1-2.5 cm from the carina, whereas the bifurcation of the left main bronchus is typically about 5 cm distal to the carina. There is considerable anatomic variation: for example, the right upper lobe bronchus will occasionally arise from the trachea itself.
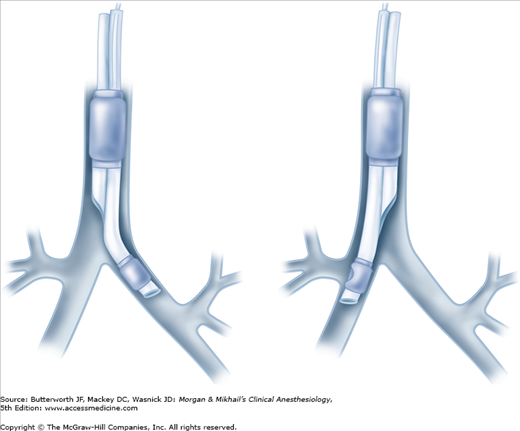
As previously noted, right-sided double-lumen tubes must have a portal through the bronchial cuff for ventilating the right upper lobe (Figure 25-6). Anatomic variations among individuals in the distance between the carina and the right upper lobe orifice will occasionally result in difficulty ventilating that lobe with right-sided tubes. A left-sided double-lumen tube can be used in most surgical procedures, irrespective of the operative side.  There are certain clinical situations in which the use of a right-sided double-lumen tube is recommended: (1) distorted anatomy of the left main bronchus by an intrabronchial or extrabronchial mass; (2) compression of the left main bronchus due to a descending thoracic aortic aneurysm; (3) left-sided pneumonectomy; (4) left-sided single lung transplantation; and (5) left-sided sleeve resection. Finally, despite concerns about right upper lobe atelectasis and potentially difficult placement, studies have failed to detect differences in clinical performance of right- and left-sided double-lumen tubes when used clinically.
There are certain clinical situations in which the use of a right-sided double-lumen tube is recommended: (1) distorted anatomy of the left main bronchus by an intrabronchial or extrabronchial mass; (2) compression of the left main bronchus due to a descending thoracic aortic aneurysm; (3) left-sided pneumonectomy; (4) left-sided single lung transplantation; and (5) left-sided sleeve resection. Finally, despite concerns about right upper lobe atelectasis and potentially difficult placement, studies have failed to detect differences in clinical performance of right- and left-sided double-lumen tubes when used clinically.
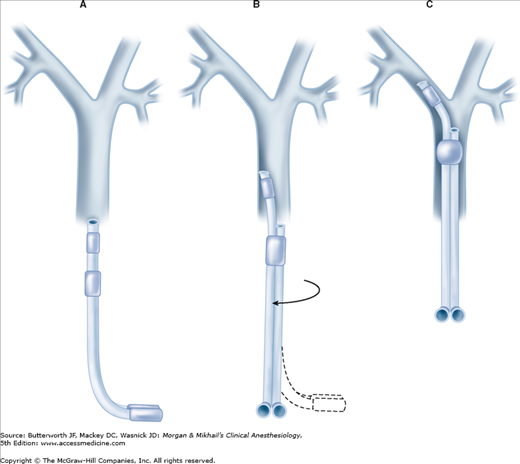
Laryngoscopy with a curved (MacIntosh) blade usually provides better intubating conditions than does a straight blade because the curved blade typically provides more room for manipulation of the large double-lumen tube. Video laryngoscopy can also be employed to facilitate tube placement. The double-lumen tube is passed with the distal curvature concave anteriorly and is rotated 90° (toward the side of the bronchus to be intubated) after the tip passes the vocal cords and enters the larynx (Figure 25-7). At this point, the operator has two options: the tube can be advanced until resistance is felt (the average depth of insertion is about 29 cm [at the teeth]), or alternatively, the fiberoptic bronchoscope can be inserted through the bronchial limb and advanced into the desired bronchus. The double-lumen tube can be advanced over the bronchoscope into the desired bronchus. Correct tube placement should be established using a preset protocol (Figure 25-8 and Table 25-2) and confirmed by flexible fiberoptic bronchoscopy. When problems are encountered in intubating the patient with the double-lumen tube, placement of a single-lumen endotracheal tube should be attempted; once positioned in the trachea, the latter can be exchanged for the double-lumen tube by using a specially designed catheter guide (“tube exchanger”).
|
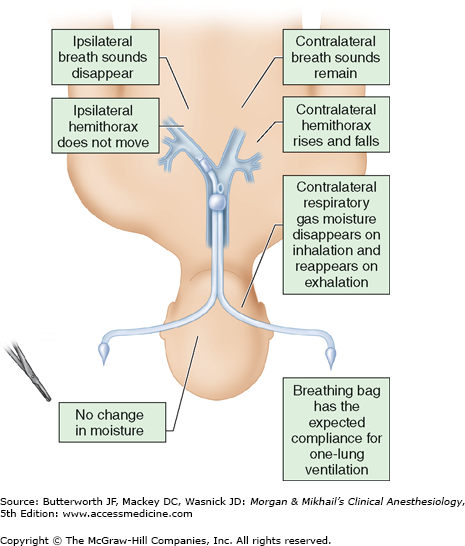
Most double-lumen tubes easily accommodate bronchoscopes with a 3.6- to 4.2-mm outer diameter. When the bronchoscope is introduced into the tracheal lumen and advanced through the tracheal orifice, the carina should be visible (Figure 25-9), and the bronchial limb of the tube should be seen entering the respective bronchus; additionally, the top of the bronchial cuff (usually colored blue) should be visible, but should not extend above the carina. If the bronchial cuff of a left-sided double-lumen tube is not visible, the bronchial limb may have been inserted sufficiently far to allow the bronchial cuff to obstruct the orifice of the left upper or lower lobe; the tube should be withdrawn until the cuff can be identified distal to the carina. The optimal position of a right-sided double-lumen tube is confirmed by placing the fiberoptic scope through the endobronchial lumen, which should show alignment of the endobronchial side portal with the opening of the right upper lobe bronchus. The bronchial cuff should be inflated only to the point at which the audible leak from the open tracheal lumen disappears while ventilating only through the bronchial lumen. Tube position should be reconfirmed after the patient is positioned for surgery because the tube may move relative to the carina as the patient is turned into the lateral decubitus position. Malpositioning of a double-lumen tube is usually indicated by failure of the operative lung to collapse, poor lung compliance, and low exhaled tidal volume. Problems with left-sided double-lumen tubes are usually related to one of three possibilities: (1) the tube tip is too distal: (2) the tube tip is too proximal: or (3) the tube is in the right bronchus (the wrong side). If the tube tip is located too distally, the bronchial cuff can obstruct the left upper or the left lower lobe orifice, and the bronchial lumen can be inserted into the orifice of the left lower or left upper lobe bronchus, respectively. When the tube is not advanced distally enough, the inflated bronchial cuff may be above the carina and also occlude the tracheal lumen. In both instances, deflation of the bronchial cuff improves ventilation to the lung and helps to identify the problem. In some patients, the bronchial lumen may be within the left upper or left lower lobe bronchus but with the tracheal opening remaining above the carina; this situation is suggested by collapse of only one of the left lobes when the bronchial lumen is clamped. In the same situation, if the surgical procedure is in the right thorax, clamping of the tracheal lumen will lead to ventilation of only the left upper or left lower lobe; hypoxia usually develops rapidly.
Right-sided double-lumen tubes can be accidentally inserted into the left main stem bronchus, inserted too distally or too proximally, or have misalignment of the endobronchial side portal with the opening of the right upper lobe bronchus. If the tube inadvertently enters the wrong bronchus, the fiberoptic bronchoscope can be used to direct it into the correct side: (1) the bronchoscope is passed through the bronchial lumen to the tip of the tube; (2) under direct vision, the tube and the bronchoscope are withdrawn together into the trachea just above the carina; (3) the bronchoscope alone is then advanced into the correct bronchus; and (4) the double-lumen tube is gently advanced over the bronchoscope, which functions as a stylet to guide the bronchial lumen into the correct bronchus.
Major complications of double-lumen tubes include: (1) hypoxemia due to tube malplacement, tube occlusion, or excessive degrees of venous admixture with one-lung ventilation; (2) traumatic laryngitis; (3) tracheobronchial rupture resulting from traumatic placement or overinflation of the bronchial cuff; and (4) inadvertent suturing of the tube to a bronchus during surgery (detected as the inability to withdraw the tube during attempted extubation).
Bronchial blockers are inflatable devices that are passed alongside or through a single-lumen tracheal tube to selectively occlude a bronchial orifice. A single-lumen tracheal tube with a built-in side channel for a retractable bronchial blocker is available. The tube is placed with the blocker fully retracted; its natural curve is such that turning the tube with the curve concave toward the right preferentially directs the bronchial blocker toward the right bronchus. Turning the tube with the curve concave toward the left usually directs the blocker toward the left bronchus. The bronchial blocker must be advanced, positioned, and inflated under direct visualization via a flexible bronchoscope.
The major advantage of a tube with an incorporated bronchial blocker is that, unlike a double-lumen tube, it does not need to be replaced with a conventional tracheal tube if the patient remains intubated postoperatively (below). Its major disadvantage is that the “blocked” lung collapses slowly (and sometimes incompletely) because of the small size of the channel within the blocker.
Another way to achieve lung separation is by using an independent bronchial blocker passed through a single-lumen endotracheal tube. There are several types of independent bronchial blockers. They come in different sizes (7Fr and 9Fr) and have a 1.4-mm diameter inner lumen. Bronchial blockers have a high-volume low-pressure cuff with either an elliptical or spherical shape. The spherical shape of the cuff facilitates adequate blockade of the right mainstem bronchus. The spherical or the elliptical cuff can be used for the left main stem bronchus. The inner lumen contains a nylon wire, which exits the distal end as a wireloop. The placement of the bronchial blocker involves inserting the endobronchial blocker through the endotracheal tube and using the fiberoptic bronchoscope and the distal loop of the guidewire to direct the blocker into a mainstem bronchus. The fiberoptic bronchoscope must be advanced beyond the bronchus opening so that the blocker enters the bronchus while it is being advanced. When the deflated cuff is beyond the entrance of the bronchus, the fiberoptic bronchoscope is withdrawn, and the blocker is secured in position. In order to obtain bronchial blockade, the cuff is fully inflated under fiberoptic visualization with 4 to 8 mL of air. The placement must be reconfirmed when the patient is placed in the lateral position. Bronchial blockers may be good choices for lung separation in intubated critically ill patients who require one-lung ventilation, patients who are difficult to intubate using direct laryngoscopy, patients with prior tracheostomies, and patients who may require postoperative mechanical ventilation. However, because bronchial blockers are more prone to dislodgement compared with double-lumen endotracheal tubes, and their small central lumens do not allow efficient suctioning of secretions or rapid collapse of the lung, some clinicians prefer not to use them.
In smaller children, an inflatable embolectomy (Fogarty) catheter can be used as a bronchial blocker in conjunction with a conventional tracheal tube (with the embolectomy catheter placed either inside or alongside the tracheal tube); a guidewire in the catheter can be used to facilitate placement. This technique is occasionally used to collapse one lung when other techniques do not work. As the embolectomy catheter does not have a communicating channel in the center, it also does not allow suctioning or ventilation of the isolated lung, and the catheter can be easily dislodged. Nonetheless, such bronchial blockers may be useful for one-lung anesthesia in pediatric patients and for tamponading bronchial bleeding in adult patients (see below).
Anesthesia for Lung Resection
Lung resections are usually carried out for the diagnosis and treatment of pulmonary tumors, and, less commonly, for complications of necrotizing pulmonary infections and bronchiectasis.
Pulmonary tumors can be either benign or malignant, and, with the widespread use of bronchoscopic sampling, the diagnosis is usually available prior to surgery. Hamartomas account for 90% of benign pulmonary tumors; they are usually peripheral pulmonary lesions and represent disorganized normal pulmonary tissue. Bronchial adenomas are usually central pulmonary lesions that are typically benign, but occasionally may be locally invasive and rarely metastasize. These tumors include pulmonary carcinoids, cylindromas, and mucoepidermoid adenomas. They often obstruct the bronchial lumen and cause recurrent pneumonia distal to the obstruction in the same area. Primary pulmonary carcinoids may secrete multiple hormones, including adrenocorticotropic hormone (ACTH) and arginine vasopressin; however, manifestations of the carcinoid syndrome are uncommon and are more likely with metastases.
Malignant pulmonary tumors are divided into small (“oat”) cell and non-small cell carcinomas. The latter group includes squamous cell (epidermoid) tumors, adenocarcinomas, and large cell (anaplastic) carcinomas. All types are more commonly encountered in smokers, but more “never smokers” die of lung cancer each year in the United States than the total number of people who die of ovarian cancer. Epidermoid and small cell carcinomas usually present as central masses with bronchial lesions; adenocarcinoma and large cell carcinomas are more typically peripheral lesions that often involve the pleura.
Symptoms may include cough, hemoptysis, wheezing, weight loss, productive sputum, dyspnea, or fever. Pleuritic chest pain or pleural effusion suggests pleural extension. Involvement of mediastinal structures is suggested by hoarseness that results from compression of the recurrent laryngeal nerve, Horner’s syndrome caused by involvement of the sympathetic chain, an elevated hemidiaphragm caused by compression of the phrenic nerve, dysphagia caused by compression of the esophagus, or the superior vena cava syndrome caused by compression or invasion of the superior vena cava. Pericardial effusion or cardiomegaly suggests cardiac involvement. Extension of apical (superior sulcus) tumors can result in either shoulder or arm pain, or both, because of involvement of the C7-T2 roots of the brachial plexus (Pancoast syndrome). Distant metastases most commonly involve the brain, bone, liver, and adrenal glands.
Lung carcinomas—particularly small cell—can produce remote effects that are not related to malignant spread (paraneoplastic syndromes). Mechanisms include ectopic hormone production and immunologic cross-reactivity between the tumor and normal tissues. Cushing’s syndrome, hyponatremia, and hypercalcemia may be encountered, resulting from secretion of ACTH, arginine vasopressin, and parathyroid hormone, respectively. Lambert-Eaton (myasthenic) syndrome is characterized by a proximal myopathy in which muscle strength increases with repeated effort (in contrast to myasthenia gravis). Other paraneoplastic syndromes include peripheral neuropathy and migratory thrombophlebitis.
Surgery is the treatment of choice to reduce the tumor burden in nonmetastatic lung cancer. Various chemotherapy and radiation treatments are likewise employed, but there is wide variation among tissue types in their sensitivity to chemotherapy and radiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree