TOPICS
PRETERM LABOR
Introduction
Prematurity is a serious cause of adverse perinatal outcome. It is a contributing factor in 75% of neonatal deaths and may result in neonatal neurologic injury.1 Preterm labor is defined as the onset of labor prior to 37 weeks’ gestation. Anesthetic management includes analgesia for labor and vaginal delivery, as well as anesthesia for cesarean delivery if indicated. Furthermore, the anesthesiologist may become involved in managing the effects of tocolytic agents.
Risk Factors for Preterm Labor
Genetic, hormonal, psychosocial, and environmental factors are believed to be associated with preterm labor. Risk factors, identified in fewer than 50% of cases, include, but are not limited to nonwhite race, low socioeconomic status, history of preterm delivery, multiple gestation, preterm premature rupture of membranes, abnormal uterine anatomy, abnormal cervical anatomy, genital or systemic infection, trauma, abdominal surgery, fetal genetic abnormalities, fetal death, and tobacco/substance use.2
Pathophysiology of Preterm Labor
Initiation of labor is complex and multifactorial, and it includes genetic as well as hormonal factors. The characteristics of both term and preterm labor include cervical dilation and effacement, increased uterine contractility, and activation of the amniochorionic membrane. In term gestation, these changes are routine conclusions to pregnancy. However, in preterm labor, these changes are initiated through pathologic mechanisms.3 During normal pregnancy, activation of the fetal hypothalamic-pituitary-adrenal axis contributes to labor initiation. Secretion of adrenocorticotropic hormone (ACTH) increases in response to release of corticotropin-releasing hormone from the hypothalamus. ACTH, in turn stimulates the adrenal glands to secrete cortisol.4 This leads to an inflammatory response resulting in increased myometrial prostaglandin, which in turn produces an increase in intracellular calcium, with subsequent initiation of uterine contractions.2 Thus, any stimulus that can initiate the inflammatory cascade of mediators can lead to uterine contractions, even prior to term of pregnancy.
Diagnosis
Preterm labor occurs at 20 to 37 weeks’ gestation. Uterine contraction frequency should be at least 4 or more in a 20-minute period, or 8 or more in a 60-minute period. There must be either ongoing cervical changes, cervical dilation of at least 2 cm, or effacement of at least 80%.2 False labor is characterized by irregular contractions that do not increase in frequency, duration, or strength. Also, there is no change in cervical dilation or effacement with false labor.3
Tocolytics versus Delivery
Not all patients with preterm labor progress to preterm delivery. Preterm delivery is associated with the potential for increased neonatal morbidity and mortality; therefore, prevention of delivery, or at least prolongation of pregnancy, is desirable. Tocolytic medications inhibit uterine contractions and should be considered for parturients at 20 to 34 weeks’ gestation with reassuring fetal status and absence of infection2 who are in preterm labor. Contraindications to tocolysis include fetal death, fetal anomalies incompatible with life, nonreassuring fetal status, chorioamnionitis or fever of unknown origin, severe hemorrhage, severe chronic hypertension or pregnancy complicated by preeclampsia3 or other maternal contraindication.
TOCOLYTICS
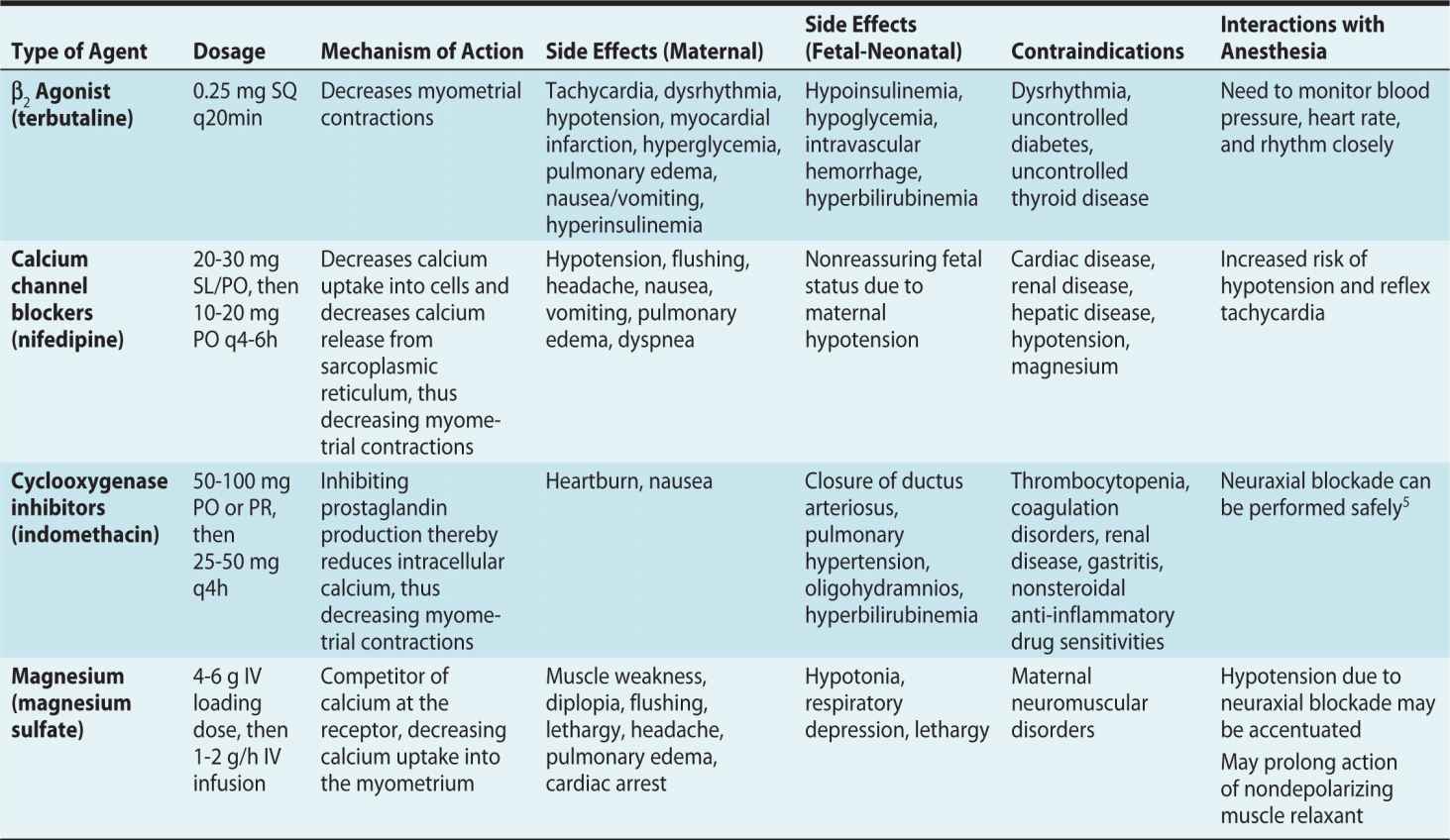
Tocolytics can decrease the frequency, strength, and duration of contractions. They do not generally stop preterm labor but may be effective in prolonging the time to delivery by 2 to 7 days. This allows time for antenatal steroid administration to promote fetal lung maturity, or for transfer to a tertiary care facility with an appropriate capacity for handling preterm births.1 Four classes of medications are used to decrease uterine contractions during preterm labor: β-adrenergic agonists, calcium channel blockers, cyclooxygenase inhibitors (nonsteroidal anti-inflammatory drugs), and magnesium. The preferred agent of choice depends on the presence of maternal comorbidities and the ability to tolerate the side effects. A summary of available agents are listed in Table 10-1.1,2,4,5
Table 10-1. Tocolytic Agents Used in Preterm Labor
SUMMARY: TOCOLYTIC AGENTS
Studies have failed to demonstrate any specific tocolytic agent that is being more efficacious than another in prolonging preterm labor.6,7 Thus, medications are chosen based on individual considerations such as maternal comorbidities, gestational age, and the potential for side effects. Magnesium and nifedipine have been reported to be the preferred first-line tocolytics in the United States.8 Nifedipine has the advantage of being an effective tocolytic with minimal side effects.6,9 Terbutaline is often avoided due to its effects on the maternal and fetal cardiovascular systems. Cyclooxygenase inhibitors have fallen out of favor due to the potential risk of ductus arteriosus closure and fetal pulmonary hypertension.
Antenatal Steroids
The purpose of antenatal steroid administration is to decrease the risk of respiratory distress syndrome (RDS) in the neonate. Fetal surfactant is composed of phospholipids that decrease the surface tension of alveolar walls and thus prevent collapse. Secretion of surfactant begins at about 24 weeks’ gestation and is completed by 34 weeks’ gestation. Therefore, mothers at gestational ages between 24 and 34 weeks who are at risk of preterm delivery within 7 days, should be considered for steroid therapy. Use of antenatal steroids before preterm delivery decreases the incidence of RDS, intraventricular hemorrhage, and neonatal death.10 Two regimens are recommended in pregnant women between 24 and 34 weeks’ gestation who are at risk for preterm delivery within 7 days: (1) two doses of betamethasone 12 mg intramuscularly at an interval of 24 hours or (2) four doses of dexamethasone 6 mg intramuscularly at an interval of 12 hours.10
Analgesia for Preterm Labor and Vaginal Delivery
Prematurity does not preclude maternal analgesia. Regional analgesia remains the most effective route of analgesia and may be beneficial in providing perineal relaxation and a controlled delivery of the fetal head. Furthermore, an early regional technique is recommended because delivery of the preterm fetus may occur at less than 10-cm cervical dilation.2 Also, the preterm fetus is at higher risk for developing hypoxia and acidosis, and a well-functioning regional analgesic can easily be converted quickly to an anesthetic should an emergency cesarean delivery may be warranted (thus avoiding the need for general anesthesia). Combined spinal-epidural (CSE) analgesia is also an efficient modality that provides rapid onset of analgesia during labor and effective anesthesia for cesarean delivery via the epidural component. Use of intrathecal opioids has been reported to be associated with fetal bradycardia in healthy neonates; this is related to sudden decrease in catecholamines and subsequent increased uterine contraction due to hyperactivity of α-adrenergic agonists or greater responsiveness to endogenous or exogenously administered oxytocin.11 The preterm fetus may not have the well-defined characteristics and responses in heart rate of a term fetus. For that reason, it is important that there be good communication among the members of the delivery team. Often, as a norm, a preterm fetus may have heart rates greater than 160 beats/min and decreased variability.2
Anesthesia for Cesarean Delivery for the Parturient in Preterm Labor
Cesarean delivery for the parturient in preterm labor is indicated for nonreassuring fetal status, history of previous cesarean delivery (not allowed to labor), and for breech presentation. It is important to obtain a comprehensive preanesthetic evaluation, paying particular attention to tocolytic side effects. For emergent and “stat” situations related to fetal heart rate abnormalities, it is important to reevaluate fetal heart rate once in the operating room because often the fetal heart rate may have improved and consideration may be given to regional as compared to general anesthesia if time allows.
REGIONAL ANESTHESIA
Neuraxial anesthesia is the preferred anesthetic technique for cesarean delivery, avoiding the airway risks associated with general anesthesia. Regional anesthesia also reduces the potential for transient fetal effects of central nervous system–depressing anesthetic agents. A single-shot or CSE anesthesia provides rapid onset of dense sensory blockade. If the fetus is breech and there has been significant cervical dilation, the lateral rather than sitting position may be preferred for initiation of neuraxial blockade to avoid umbilical cord prolapse. Neonatal resuscitation may be required; thus, a neonatologist should be present for delivery.
GENERAL ANESTHESIA
General anesthesia is usually reserved if there is rapid dilation in a preterm breech with head entrapment; if there is dire nonreassuring fetal status where any delay may adversely affect outcome; or where there may be maternal contraindication to neuraxial blockade, usually related to the side effects of tocolytics. Rapid sequence induction is performed with standard induction agents (thiopental, propofol, ketamine, or etomidate) and muscle relaxant (succinylcholine at 1-1.5 mg/kg). If the patient has been given magnesium, avoid nondepolarizing neuromuscular blockers, or administer a small dose, because the effects will be prolonged. The use of a neuromuscular monitor to measure the depth of blockade and guide drug administration should be routine. There has been concern regarding the potential for neurocognitive effects in the newborn related to in utero exposure to anesthetic agents. All the anesthetic agents have been implicated, with the exception of local anesthetics as used in regional obstetric anesthesia. Thus, wherever possible, we believe that the use of regional is preferred to general anesthesia for both maternal and fetal considerations. However, it is also our belief that fetal hypoxia and acidosis are far worse for the developing fetal brain and in dire situations, if a preexisting regional anesthetic is not in place, general anesthesia may be required simply because it can be induced quickly.12
MULTIPLE GESTATION
Multiple gestation refers to a pregnancy with twins or higher order multiples (eg, triplets, quadruplets).
Incidence
In the United States, the twin birth rate rose 70% between 1980 and 2004. Since then the twin birth rate has been stable.13 In 2005, twin births represented 3% of all births and triplets and higher order multiples accounted for 0.2% of all births. In 2008, the twin birth rate in the United States was 32.6 per 1000 births and the rates of higher order multiple births (triplets, quadruplets, quintuplets, sextuplets and septuplets) was 147.6 per 100,000 births.13 The increase in incidence of multiparity may be related to growth in assisted reproductive therapies (in vitro fertilization, ovulation-inducing drugs, and artificial insemination) and pregnancy in older women.
Twin Pregnancy
There are two types of twin pregnancies: dizygotic (66%) (more common) and monozygotic (approximately 30%). In the case of dizygotic twins, two separate ova are fertilized. Dizygotic twins have separate amnions, chorions, and placentae. Monozygotic twins develop from a single fertilized ovum, which splits into two distinct individuals after conception. Twinning (splitting) cannot occur beyond 15 days after fertilization. An early splitting (ie, within 3 days after fertilization) produces separate chorions and amnions. The twins have separate placentas but can also have a single fused one. Approximately 30% of monozygotic twins are dichorionic diamniotic. Splitting between 4 and 8 days after fertilization results in monozygotic monochorionic diamniotic placentation (Figure 10-1). Approximately 70% of monozygotic twins are monochorionic diamniotic. If splitting occurs 8 to 13 days after fertilization, monochorionic monoamniotic placentation occurs. Only 1% of (monozygotic) twins have this form of placentation. If splitting occurs between 13 and 15 days after fertilization, the fertilized ovum splits only partially, resulting in conjoined twins with a monochorionic monoamniotic placenta.
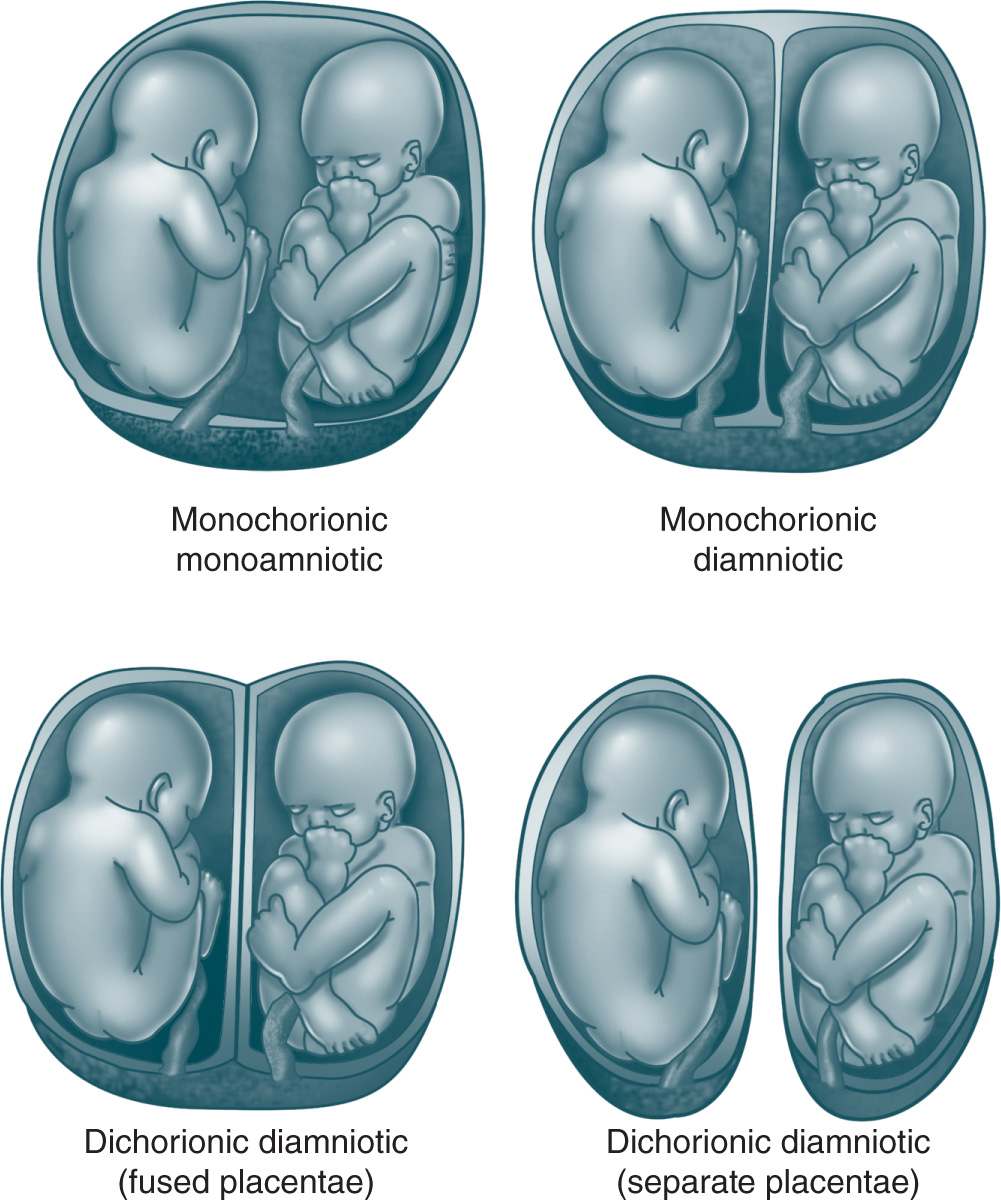
Figure 10-1. Placentation in twin pregnancies. From Cleary-Goldman J, Chitkara U, Berkowitz R. Multiple gestations. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: Normal and Problem Pregnancies. 5th ed. New York, NY: Churchill Livingstone; 2007:736.
Is It Important to Distinguish Monochorionic From Dichorionic Twins?
Yes, this is because monochorionic twin pregnancies have a much higher rate of complications than dichorionic twin pregnancies. The placentae of nearly all monochorionic twin (monochorionic monoamniotic or monochorionic diamniotic) have vascular communications. Most of these anastomoses are of little fetal consequence because distribution of blood supply is well balanced. However, deeper vascular anastomoses can result in net transfusion of blood from one twin (the donor) to the other twin (the recipient), leading to twin-twin transfusion syndrome (TTTS). The donor twin is usually smaller, suffers from anemia, hypovolemia, and produces less urine. Because urine is the main component of amniotic fluid, the amniotic fluid surrounding the donor will decrease. Severe oligohydramnios can result in the “stuck twin phenomena” (ie, the twins appear “stuck” up against the wall of the uterus in a fixed position).14 The recipient twin may also develop complications; he or she receives an excess of blood from the donor twin and may develop polyhydramnios, polycythemia, and cardiac failure. In contrast to the donor twin, polyhydramnios develops in the sac because of increased fetal urine output.
TTTS complicates about 15% of monochorionic twin pregnancies, usually between 18 and 26 weeks’ gestation.14 Complications include significant neurologic injury to the fetus and even fetal death. Current therapeutic options for TTTS include drainage of excessive fluid (amniocentesis), surgical separation of connecting vessels in the placenta by means of laser (selective laser photocoagulation of communicating vessels [SLPCV]), amniotic septostomy (creating connection between amniotic membrane), and selective feticide. The goal of amniocentesis is to decrease the likelihood of preterm labor by reducing the amniotic fluid volume in the sac of the recipient twin. In contrast, septostomy equilibrates pressures between the two amniotic cavities.15
Is It Possible to Distinguish Between Monochorionic and Dichorionic Twins?
The distinction between monochorionic and dichorionic twins can be made on ultrasound scan with 100% accuracy early in pregnancy (ie, before the 14th week of pregnancy). Later on in pregnancy, it becomes far more difficult and will depend on the interpretation of membrane thickness.15
Risks of a Multiple Gestation
FETAL COMPLICATIONS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








