Anesthesia for Neurosurgery
An understanding of neuroanatomy is essential for all who care for patients with disease of the central nervous system (CNS) (Dagal A, Lam AM. Anesthesia for neurosurgery. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Ortega R, Stock MC, eds. Clinical Anesthesia. Philadelphia: Lippincott Williams & Wilkins; 2013:996–1029).
I. Neuroanatomy
The brain and spinal cord are surrounded by protective but nondistensible bony structures that surround them and provide protection.
The intracranial volume is fixed, thereby providing little room for anything other than the brain, cerebrospinal fluid (CSF), and blood contained in the cerebral vasculature. It is in the context of the restrictive nature of the space in which the CNS is housed that all interventions must be considered.
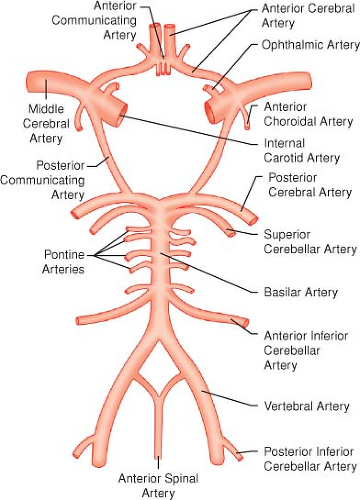
The anterior cerebral circulation originates from the carotid artery, the posterior circulation results from the vertebral arteries, and the system of collateralization is known as the circle of Willis (Fig. 36-1).
The spinal column is the bony structure made up of the seven cervical, 12 thoracic, and five lumbar vertebrae, as well as the sacrum.
The spinal cord exits the skull through the foramen magnum and enters the canal formed by the vertebral bodies. In adults, the spinal cord typically ends at the lower aspect of the first lumbar vertebral body.
The anterior spinal artery arises from the vertebral arteries and supplies the anterior two thirds of the spinal cord. This vessel runs the length of the cord, receiving contribution from radicular arteries via intercostal vessels. The artery of Adamkiewicz is the most important radicular vessel.
The posterior third of the cord is supplied by two posterior spinal arteries, which arise from the vertebral arteries and also receive contribution from radicular arteries.
II. Neurophysiology
Cerebral metabolism is directly related to the number and frequency of neuron depolarizations (activity or stimulation increases the metabolic rate). Cerebral blood flow (CBF) is tightly coupled to metabolism.
The CSF occupies the subarachnoid space, providing a protective layer of fluid between the brain and the tissue that surrounds it. It maintains a milieu in which the brain can function by regulating pH and electrolytes, carrying away waste products, and delivering nutrients.
Intracranial pressure (ICP) is low except in pathologic states. The volume of blood, CSF, and brain tissue must be in equilibrium. An increase in one of these three elements, or the addition of a space-occupying lesion, can be accommodated initially through displacement of CSF into the thecal sac but only to a small extent. Further increases, as with significant cerebral edema or accumulation of an extradural hematoma, quickly lead to a marked increase in ICP because of the low intracranial compliance (Fig. 36-2).
Many factors affect CBF because of their effect on metabolism. (Stimulation, arousal, nociception, and mild hyperthermia elevate metabolism and flow, and sedative–hypnotic agents and hypothermia decrease metabolism and flow.)
A number of other factors govern CBF directly without changing metabolism.
A potent determinant of CBF is PaCO2. CBF changes by approximately 3% of baseline for each 1 mm Hg of change in PaCO2 (Fig. 36-3).
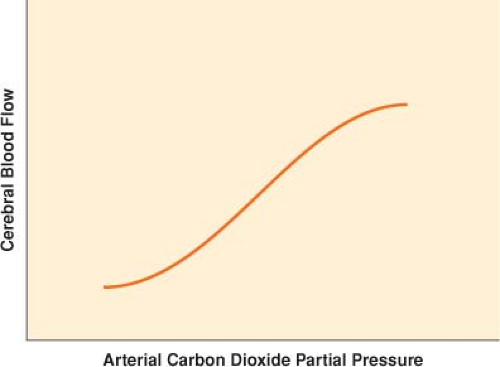
Figure 36-3. Cerebrovascular response to a change in arterial carbon dioxide partial pressure (PaCO2) from 25 to 65 mm Hg.
As CBF changes, so does cerebral blood volume (CBV), which is the reason hyperventilation can be used for short periods of time to relax the brain or to decrease ICP. This effect is thought to be short lived (minutes to hours) because the pH of CSF normalizes over time and vessel caliber returns to baseline.
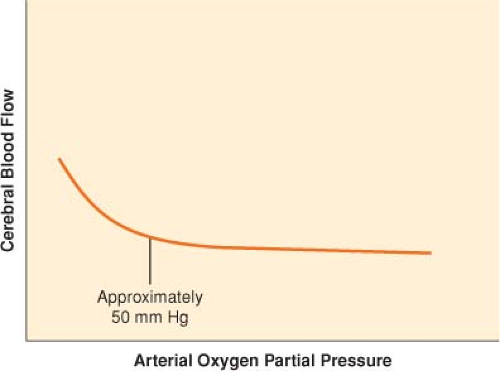
In contrast to PaCO2, PaO2 has little effect on CBF except at abnormally low levels (Fig. 36-4). When PaO2 decreases below 50 mm Hg, CBF begins to increase sharply.
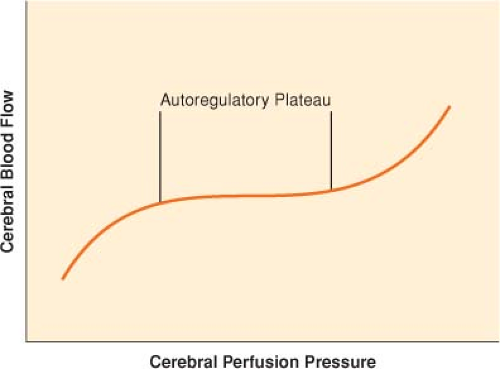
CBF remains approximately constant despite modest swings in arterial blood pressure (autoregulation) (Fig. 36-5).
As cerebral perfusion pressure (CPP), defined as the difference of mean arterial pressure (MAP) and ICP, changes, cerebrovascular resistance (CVR) adjusts in order to maintain stable flow.
The range of CPP over which autoregulation is maintained is termed the autoregulatory plateau. Although this range is frequently quoted as a MAP range of 60 to 150 mm Hg, there is significant variability between individuals, and these numbers are only approximate.
At the low end of the plateau, CVR is at a minimum, and any further decrease in CPP compromises CBF.
At the high end of the plateau, CVR is at a maximum, and any further increase in CPP results in hyperemia.
Anesthetic Influences
Anesthetic agents have a variable influence on CBF and metabolism, carbon dioxide reactivity, and autoregulation.
Inhalation anesthetics tend to cause vasodilation in a dose-related manner but do not per se uncouple CBF and metabolism. Thus, the vasodilatory influence is opposed by a metabolism-mediated decrease in CBF.
The resultant effect is that during low-dose inhalation anesthesia, CBF is either unchanged or slightly increased. Compared with other inhaled agents, sevoflurane in clinically relevant doses does not increase CBF. Furthermore, sevoflurane is associated with profound regional and global reductions in cerebral metabolic rate.
Intravenous (IV) agents, including thiopental and propofol, cause vasoconstriction coupled with a reduction in metabolism. Ketamine, on the other hand, increases CBF and metabolism.
Cerebrovascular carbon dioxide reactivity is a robust mechanism and is preserved under all anesthetic conditions. Cerebral autoregulation, on the other hand, is abolished by inhalation agents in a dose-related manner but is preserved during propofol anesthesia.
III. Pathophysiology
The homeostatic mechanisms that ensure protection of the brain and spinal cord, removal of waste, and delivery of adequate oxygen and substrate to the tissue can be interrupted through a multitude of mechanisms (Table 36-1).
Table 36-1 Events that May Interrupt Homeostatic Mechanisms for Brain Protection | ||||
|---|---|---|---|---|
|
Table 36-2 Monitoring Central Nervous System Function | |||
|---|---|---|---|
|
IV. Monitoring
The integrity of the CNS needs to be evaluated intraoperatively with monitors that specifically detect CNS function, perfusion, or metabolism.
Influence of Anesthetic Technique (Table 36-5)
V. Cerebral Perfusion
Although adequate CBF does not guarantee the well-being of the CNS, it is an essential factor in its integrity.
Laser Doppler flowmetry requires a burr hole and measures flow in only a small region of the brain.
Transcranial Doppler ultrasonography (TCD) is a noninvasive monitor for evaluating relative changes in flow through the large basal arteries of the brain (often flow velocity in the middle cerebral artery) (Fig. 36-6).
Table 36-3 Electroencephalogram Frequencies
Frequency (Hz)
Description
Delta
0–3
Low frequency, high amplitude
Present in deep coma, encephalopathy, deep anesthesia
Theta
4–7
Not prominent in adults
May be seen in encephalopathy
Alpha
8–12
Prominent in the posterior region during relaxation with the eyes closed
Beta
>12
High frequency, low amplitude
Dominant frequency during arousal
In addition to the measurement of flow velocity, TCD is useful for detecting emboli (Fig. 36-7).
Specific applications for intraoperative use of TCD include carotid endarterectomy (CEA), nonneurologic surgery and cerebral autoregulation in patients with traumatic brain injury (TBI), and surgical procedures requiring cardiopulmonary bypass.
An important use of TCD is to monitor the development of vasospasm in patients who have experiences a subarachnoid hemorrhage (SAH).
Intracranial Pressure Monitoring
Although monitoring ICP does not provide direct information about CBF, it allows calculations of CPP (the difference between MAP and ICP).
Within a physiologic range of CPP, CBF should remain approximately constant. A CPP that is too low results in
cerebral ischemia, and a CPP that is too high causes hyperemia.
Table 36-4 Indications for Electroencephalographic Monitoring
During anesthesia
Carotid endarterectomy
Cardiopulmonary bypass
Cerebrovascular surgery (temporary clipping, vascular bypass)
Intensive care unit
Barbiturate coma for patients with traumatic brain injury
Subclinical seizures suspected
Table 36-5 Influence of Anesthetic Technique on Central Nervous System Monitoring
Inhalation agents (including nitrous oxide) generally have more depressant effects on evoked potential monitoring than IV agents.
Whereas cortical evoked potentials with long latency involving multiple synapses (SSEP, VEP) are exquisitely sensitive to the influence of anesthetic, short-latency brain stem (BAEP) and spinal components are resistant to anesthetic influence.
Monitoring of MEP and cranial nerve EMG in general preclude the use of muscle relaxants. Use of a short-acting neuromuscular blocking agent for the purpose of tracheal intubation is acceptable.
MEP is exquisitely sensitive to the depressant effects of inhalation anesthetics, including nitrous oxide. Total IV anesthesia without nitrous oxide is the recommended anesthetic technique for monitoring of MEP.
Opioids and benzodiazepines have negligible effects on recording of evoked potentials.
Propofol and thiopental attenuate the amplitude of virtually all modalities of evoked potentials but do not obliterate them.
During crucial events when part of the central neural pathway is specifically placed at risk by surgical manipulation, as in placement of a temporary clip during aneurysm surgery, change in “anesthetic depth” should be minimized to avoid misinterpretation of the changes in evoked potential.
BAEP = brain stem auditory evoked potential; IV = intravenous; MEP = motor evoked potential; SSEP = somatosensory evoked potential; VEP = visual evoked potential. 
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree