Fig. 6.1
Stress and stress reaction. ACTH adrenocorticotropic hormone, ADH antidiuretic hormone
Activation of the sympathoadrenergic system with release of catecholamines is an immediate and active response to the stressor; release of the antidiuretic hormone is also a component of the immediate stress response.
In contrast, activation of the hypothalamus-hypophysis-adrenal cortex axis focuses on tolerance and adaptation and, with a protracted course, is to be seen as a sign of loss of control in the confrontation with the stressor.
Later concepts emphasize organic responses to interior and exterior stressors that serve to maintain homeostasis [6]. A distinction is made between homeostatic systems in the narrow sense (e.g., oxygen concentration in the blood, body temperature) that allow changes only within very narrow limits and so-called allostatic systems that allow a wider range of responses. This is described as “maintaining stability through change (allostasis)” [7]. Allostasis as a basic function of bodily stress modulation thus reacts quickly via the sympathoadrenergic system and slowly via the hypothalamus-hypophysis-adrenal cortex axis.
Afferent neuronal stimuli and humoral factors are of great importance in modulating the surgical stress reaction.
The neural facilitation of the stress response to tissue trauma is explained by afferent impulses from nociceptive, somatosensitive, and sympathetic pathways, whereby the relative importance of the somatosensitive nervous system in comparison with the sympathetic nervous system remains unclear. The nociceptive afferents modify the neuroendocrine function of the hypothalamus and trigger the endocrine surgical stress response. A stress reaction can, however, also be induced by physiological factors (e.g., anxiety).
Humoral mediators of the stress response include prostaglandins, bradykinin, substance P, histamine, and serotonin, which are released upon tissue trauma. Among the mediators secreted by macrophages, interleukin-1 and tumor necrosis factor-α are especially important [8].
For all trauma patients – as for all critically ill patients – the effects of analgesia and anesthesia on the stress reaction must be borne in mind. The acutely injured patient is in the “fight or flight” stage, directly confronted with the trauma-induced stressors. Because the life-preserving stress reaction depends on catecholamines, the effect of anesthetics on the sympathoadrenergic system demands special attention. The goal of anesthesia and analgosedation is to preserve the stress reaction without suppression or overactivity.
6.1.3 Basic Concepts in Anesthesiology
6.1.3.1 Definitions
The following definitions will be used [9]:
Anesthesia is the Greek-Neo Latin word for insensitivity or lack of sensation and applies not only to the state of an iatrogenically induced reversible insensitivity that aims to make an intervention possible, but also to a medical procedure to induce such a state.
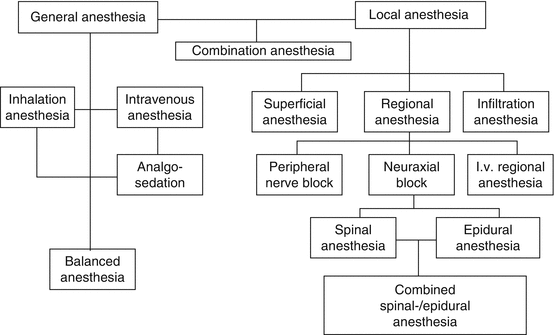
General anesthesia affects the entire organism, while local anesthesia is limited to particular areas.
Narcosis (Greek: torpor) is general anesthesia with central exclusion of pain and consciousness induced by anesthetics. “Narcosis” is synonymous with “gene-ral anesthesia” and extends the term anesthesia to cover exclusion of consciousness or hypnosis while simultaneously disallowing the term partial narcosis that is sometimes used (e.g., for spinal anesthesia).
“Narcosis” expands the term “anesthesia,” while “analgesia” limits it to the pain component by excluding sensitivity to position, touch, and temperature. Analgesia eliminates sensitivity to pain, producing painlessness. By definition, a properly “narcotized” patient cannot sense pain, therefore the term “analgesia” in the context of narcosis is problematic. Because a lack of specific inhibition of the nociceptive system in a narcotized patient is mentally and physically noxious, “antinociception” for specific blockade of the nociceptive system [10] is an essential component of adequate general anesthesis.
Local anesthesia, or more precisely, local insensitivity, with its sequence of sympathetic, sensory, and when necessary, motor blockade, is more than analgesia and thus a form of anesthesia. Local anesthesia implies regional exclusion of pain in the area of the nerve endings or nerve tracts without affecting consciousness.
6.1.3.2 Components of Anesthesia
Traditionally, anesthesia comprises three main components (Fig. 6.2):
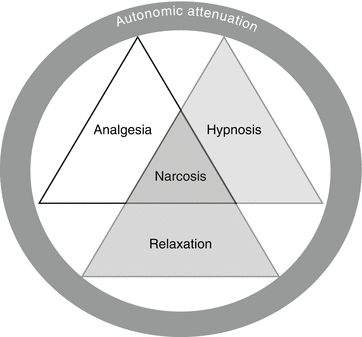

Fig. 6.2
Components of anesthesia – the specific effect sought should always be borne in mind
Analgesia as exclusion of pain only
Hypnosis as loss of consciousness
Attenuation of autonomic nervous activity
Muscle relaxation is a further component that is usually required for surgery. A clear distinction cannot be made between analgesia and hypnosis, as the respective medications interact. Analgesia and hypnosis have attenuation of autonomic nervous activity in common as an additional effect.
6.1.4 Anesthetics and Anesthetic Procedures
Anesthetics, in the broader sense, are all those medications that are specifically used to induce general or local anesthesia. The substances in their main groups of general anesthetics and local anesthetics are classified according to their mode of application:
Local anesthetics are applied to the immediate vicinity of their target area.
General anesthetics are classified according to their mode of application as inhalation and intravenous anesthetics.
Inhalation anesthetics include volatile substances such as sevoflurane or desflurane and the gases nitrous oxide (laughing gas, N2O) and xenon.
Intravenous, better described as injectable anesthetics, because some may be administered intramuscularly, are the hypnotics, sedatives and analgesics, and ketamine.
Because of their lack of analgesic and hypnotic effect, muscle relaxants can only be considered as anesthetics in the widest sense.
6.2 Preclinical Analgesia and Anesthesia
6.2.1 Medications
6.2.1.1 General
For medical and logistic reasons, only a few tried and true medications are suitable for the preclinical situation (Table 6.1). In regard to the administration of anesthesia, the concrete effect (analgesia or sedation) that is desired of the respective medication must be taken into account (Fig. 6.2).
Table 6.1
Medications for analgesia, sedation, and anesthesia
Medication | Indications | Dosage | RAD |
|---|---|---|---|
Metamizole | Analgesic for mild to moderate pain | 6–12.5 (−30) mg/kg BW IV | 0.5–1.0 (−2.5) g IV |
Morphine | Analgesic for severe pain | 0.05–0.1 mg/kg BW IV | 4–8 mg IV |
Fentanyl | Highly potent analgesic for TIVA with ventilation | Analgesia with spontaneous respiration (cave respiratory depression) 0.6–1.8 μg/kg BW IV | 0.05–0.15 mg IV |
For analgosedation 3–15 μg/kg BW/h | up to 1.0 mg/h | ||
Initial TIVA 1.25–3.75 μg/kg BW IV | 0.1–0.3 mg IV | ||
Esketamine | Analgesic and anesthetic | Analgesia 0.125–0.25 mg/kg BW IV (if needed 0.25–0.5 mg/kg BW i.m.) | 10–20 mg IV (if necessary 20–40 mg i.m.) |
Analgosedation with spontaneous respiration 0.3–0.5 mg/kg BW/h | 25–40 mg/h IV | ||
Analgosedation of ventilated patients 0.3–1.5 mg/kg BW/h | 25–125 mg/h | ||
Anesthesia 0.5–1.0 mg/kg BW IV (if necessary 2.5 mg/kg BW IM) | 40–80 mg IV (if necessary 200 mg IM) | ||
Etomidate | Induction for generally stable patients | 0.25–0.5 mg/kg BW IV | 20–40 mg IV |
Midazolam | Standard sedative for sedation, analgosedation and TIVA | Sedation 0.03–0.1 mg/kg BW IV | Boluses of 1–2 mg |
Induction 0.1–0.2 mg/kg BW IV | 7.5–15 mg IV | ||
Succinylcholine | Depolarizing muscle relaxant for rapid induction | 1.0–1.5 mg/kg BW IV | 100 mg IV |
During embryogenesis and the early fetal period (until about the 16th week of gestation), analgesics and anesthetics are contraindicated except in special cases.
Metamizole is contraindicated in the first and third trimesters; in the second trimester it is limited to exceptional cases. Inhibition of prostaglandin can lead to early closure of ductus arteriosus in the newborn.
Morphine, fentanyl, and esketamine (the latter only in high doses >1 mg/kg body weight (BW)) administered to the mother shortly before birth can cause respiratory depression in the newborn.
Midozalam administered to the mother in high doses shortly before birth can cause “floppy infant” syndrome with flaccid muscle tone and respiratory depression.
Use of analgesics and anesthetics during lactation is generally not at issue because the mother’s illness will usually preclude nursing.
The abbreviation RAD used below indicates the recommended dose for an adult weighing about 75 kg, but the dose should be adjusted to the individual patient.
6.2.1.2 Metamizole
Metamizole is a pyrazolone derivative for analgesia for mild to moderate pain (e.g., soft-tissue injuries) with antipyretic and anti-inflammatory properties.
The effect begins within a few minutes and lasts for about 2 h. Side effects (SE) are decrease in blood pressure and tachycardia (that can lead to shock) when administered too quickly intravenously (IV).
The single analgesic dose is 6–12.5 mg/kg BW IV (RAD 0.5–1.0 g).
For severe pain, up to 30 mg/kg BW (2.5 g) is injected IV.
6.2.1.3 Morphine
Morphine is the standard analgesic for extremely severe pain.
The effect sets in within minutes and lasts about 4 h. SEs are respiratory depression, nausea, vomiting, and release of histamine with hypotonia. Naloxone is available as a specific antagonist.
The individual dose is 0.05–0.1 mg/kg BW IV (RAD 4–8 mg).
6.2.1.4 Fentanyl
Fentanyl is a highly potent synthetic μ-receptor antagonist for profound analgesia with total IV anesthesia (TIVA) and airway management. It can also be used for analgesia with spontaneous breathing; however, that is off-label use.
It is effective within 2–3 min and remains so for 20–40 min. The most important SE is a potentially life threatening respiratory depression. Naxolone is the specific antagonist.
For analgesia with spontaneous breathing, 0.6–1.8 μg/kg BW (RAD 0.05–0.15 mg) are administered IV in selected cases (cave respiratory depression, if necessary use breathing on command or a breathing mask).
Depending on the patient’s general condition, 1.25–3.75 μg/kg BW (RAD 0.1–0.3 mg) is injected IV.
6.2.1.5 Esketamine and Ketamine
Esketamine is the dextrorotatory isomer of the ketamine racemate. It has double the analgesic and anesthetic potency of the racemic mixture as well as a higher elimination rate with shorter time to awakening. The dosages for esketamine are given below; if the ketamine racemate is used, the dose should be doubled.
Esketamine is an anesthetic with a strong analgesic and weak hypnotic effect. Depending on the dosage, it can be used for analgesia and analgosedation as well as anesthesia that, because of its typical effects, is termed dissociative anesthesia.
Centrally modulated activation of the sympathetic nervous system by the substance increases blood pressure, heart rate (HR), and myocardial oxygen demand. Spontaneous breathing is usually maintained. After IV administration and after a circulation time, the analgesic effect sets in and persists for some 15 min. After intramuscular (IM) injection, the effect commences within 2–5 min and lasts for up to 30 min.
No relevant increase in intracranial pressure (ICP) is to be expected with controlled normoventilation. SE include dreams and nightmares and hypersalivation. It is contraindicated for patients with hypertension, coronary heart disease, preeclampsia, and eclampsia. Dream reactions and excessive circulatory effects can be diminished or avoided with combination with midazolam; atropine combats hypersalivation.
For analgesia, 0.125–0.25 mg/kg esketamine (RAD 10–20 mg) is injected IV; this can be followed by half of the initial dose when necessary. If there is no venous access, 0.25–0.5 mg/kg BW (RAD 20–40 mg) can be injected IM.
For analgosedation with spontaneous breathing, 0.3–0.5 mg/kg BW/h esketamine (RAD 25–40 mg/h) is injected IV by pump, or an IV drip with 0.5 mg esketamine/ml (1 ml = 20 drops) can be used. Dosage depends on the effect. For sedation, midazolam is administered in a lower fractionated dose (1–2 mg) IV, or alternatively, 0.03 mg/kg BW/h (RAD 2.5 mg/h) is administered by injection pump. Before the IV drip is started, adequate analgesia and sedation should be secured with IV boluses of esketamine and when needed, midazolam.
For monoanesthesia for rapid sequence induction (RSI) of patients in a poor general condition (e.g., in shock) 0.5 mg/kg BW (RAD 40–50 mg) is injected IV.
For TIVA, esketamine is typically combined with midazolam. For RSI as is often necessary for emergency medical service, depending on the patient’s condition, first up to 0.1 mg/kg BW midazolam (RAD 8 mg) is injected IV, followed by 0.5–1.0 mg/kg BW esketamine (RAD 40–80 mg) and when necessary, 1.5 mg/kg BW succinylcholine (RAD 100 mg). When necessary, further injection of half of the initial dose of esketamine can be administered, but further midazolam injections are seldom required.
For IM induction – as a last resort when there is no venous access – about 2.5 mg/kg BW esketamine (RAD 200 mg) is injected along with 0.01 mg/kg BW atropine (up to 0.5 mg). Anesthesia sets in within a few minutes and a venous access should be established immediately.
For management of special cases of uncooperative patients, 1.25–2.5 mg/kg BW esketamine (RAD 100–200 mg) is injected IM to enable venipuncture and further measures.
6.2.1.6 Etomidate
Etomidate is an induction hypnotic without analgesic potency or significant effect on the circulatory system. It is used for induction in generally stable patients.
For induction, depending on the patient’s general condition 0.25–0.5 mg/kg BW (RAD 20–40 mg) is injected IV. Typically, fentanyl or esketamine is also needed.
Etomidate suppresses cortisol (hydrocortisone) synthesis in the adrenal cortex for approximately 1 day and a single dose increases mortality in septic patients.
6.2.1.7 Midazolam
Midazolam is a benzodiazepine with sedating, anxiolytic, and amnestic effects. It is used alone as a sedative or in combination with esketamine or fentanyl for TIVA.
The effect is seen quickly and usually lasts about 30 min. There can be severe respiratory depression in elderly patients and those in poor general condition. With careful dosage, cardiovascular SEs should not be expected. Flumazenil is the specific antagonist.
For sedation, a fractionated IV dose of small boluses of 1–2 mg is administered until the patient slurs his or her speech or is sleepily responsive (total dose 0.03–0.1 mg/kg BW).
For induction, 0.1–0.2 mg/kg BW (RAD 7.5–15 mg) is injected combined with esketamine or fentanyl.
6.2.1.8 Succinylcholine
Succinylcholine is a depolarizing muscle relaxant that has the shortest onset time (30–45 s) and the shortest action time of all muscle relaxants.
SEs are sinus bradycardia, other arrhythmias (especially in children), triggering of malignant hyperthermia (MH), and increased serum potassium. Because of the danger of hyperkalemia, it is contraindicated in patients with neuromuscular diseases (particularly extensive paralysis), longer immobilization, and predisposition to MH.
Dosage is 1.0–1.5 mg/kg BW IV (RAD 100 mg).
6.2.1.9 Vecuronium
Vecuronium is a moderately long-acting, non-depolarizing muscle relaxant without any particular SEs that is easily stored in its dry form.
The initial dose is 0.1 mg/kg BW IV (RAD 8 mg) followed by 0.025 mg/kg BW IV (RAD 2 mg) if necessary.
Other moderately long-acting relaxants are also suitable. In all, the indications for these substances are limited (e.g., prevention of bucking and pressing in spite of adequate anesthesia with head injury).
6.2.2 Practical Procedure
6.2.2.1 General Aspects of Analgesia and Anesthesia
In the preclinical situation, analgesia or anesthesia should be applied conservatively and only after careful consideration. Anxious nontreatment and noncritical overtreatment are both to be avoided.
The following basic aspects should be borne in mind:
Whenever possible, all medications should be administered by drip through a safe venous access.
Analgesics should be titrated, beginning with one half the normal dose or less, depending on the patient’s condition. With opioids and benzodiazepines, the physician should wait until the medication takes effect before administering additional doses, and this requires patience. Anesthesia in contrast generally requires RSI with high doses of anesthetics to preclude defensive reflexes and movements.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree