CHAPTER 13 Adjunctive Pharmacologic Therapies in Acute Myocardial Infarction
ADJUNCTIVE PHARMACOLOGIC therapies play an important role in the management of patients with acute myocardial infarction (MI). It became increasingly clear 2 decades ago that primary management of patients with acute MI should be aimed at the occlusive coronary thrombus,1 and should include early reperfusion therapy with thrombolytic agents or mechanical devices.2,3 Because not all patients could be reperfused within 2 hours of onset of coronary thrombosis,4 however, the goal of early and complete reperfusion could not be achieved in everyone.5,6 Late reperfusion is associated with myocardial stunning or persistent left ventricular dysfunction,7,8 and early but incomplete reperfusion might be harmful.6
Evidence accrued during the last 2 decades supports the use of adjunctive pharmacologic therapies in addition to therapies used to reperfuse directly the infarct-related artery and tissue (i.e., conjunctive) for managing acute MI patients.9–12 These adjunctive therapies should be considered as alternative therapy for patients in whom thrombolytics are contraindicated, for widening the time window for reperfusion when therapy cannot be instituted early, for reducing reperfusion injury in patients given late reperfusion therapy, and for achieving complete reperfusion. The ultimate goal of therapy for acute MI, whether primary or adjunctive, is to preserve myocardium and myocardial geometry and function, decreasing cardiovascular morbidity and mortality. Specifically, the aims of adjunctive therapy are to limit consequences of ischemia or infarction, optimize healing, and reduce adverse and recurrent events.
The scope of post-MI adjunctive therapy has expanded as a result of evidence accrued over the last decade related to the following five main factors: (1) the redefinition of acute coronary syndromes to include unstable angina, non–ST segment elevation MI (NSTEMI), and ST segment elevation MI (STEMI)13–15; (2) the implementation of troponin as the preferred novel biomarker of necrosis16; (3) the implementation of novel therapeutic strategies for limiting adverse remodeling during and after healing post-MI17–25; (4) recognition that MI is the central event in the cardiovascular disease continuum extending from risk factors and coronary atherosclerosis to heart failure and death26; and (5) recognition of the higher risk of elderly patients with MI.27,28
Acute coronary syndromes result from plaque disruption leading to thrombus formation.29 Most patients with STEMI have an occlusive thrombus1,30 and develop a Q wave MI (previously termed transmural), whereas most patients with NSTEMI have a nonocclusive or mural thrombus and develop non–Q wave MI (previously termed nontransmural or subendocardial).31–33 A few patients with ST segment elevation may develop non–Q wave MI, and a few with non–ST segment elevation may develop Q wave MI. More patients present with NSTEMI than STEMI, and NSTEMI can be difficult to differentiate from unstable angina on presentation. Transmural or Q wave MIs, or STEMIs, result in more severe ventricular remodeling and dysfunction,17,34,35 however, and high mortality and morbidity.33 Early and successful reperfusion interrupts the “march to necrosis” and transmural extension as a “wavefront” from endocardium to epicardium,36 preserving most epicardial and some subendocardial myocardium and extracellular matrix and limiting ventricular remodeling.19–21
In the era before reperfusion, most MI studies focused on STEMI or Q wave MI. Ideally, all STEMI patients should receive immediate pharmacologic or catheter-based reperfusion therapy,9–11 whereas NSTEMI patients are not considered candidates for immediate catheter-based reperfusion and should have received anti-ischemic, antiplatelet, and antithrombotic therapy, and may subsequently require catheter-based therapy.12 Patients with new bundle branch block are considered candidates for reperfusion based on fibrinolytic trial data.37
In a national registry of 240,980 patients with acute MI from 1073 hospitals in the United States during 1990-1993, only 35% received thrombolytic therapy.4 The patients who were given thrombolytics in the study4 also received concomitant pharmacotherapy with intravenous heparin (96.9%), aspirin (84%), intravenous nitroglycerin (76%), oral β-blockers (36.3%), calcium channel blockers (29.5%), and intravenous β-blockers (17.4%). In the study,4 65% of the patients were not given thrombolytic therapy and were treated entirely with adjunctive pharmacologic therapies.
Use of reperfusion therapy in acute MI and adjunctive therapy after MI have increased over the last decade. In one registry from 1994-2003,38 nearly 90% of STEMI patients received acute reperfusion. In another registry of NSTEMI and STEMI patients receiving acute percutaneous coronary intervention (PCI) during 1999-2004,39 the discharge medications in the two groups were angiotensin-converting enzyme (ACE) inhibitor, 50.3% versus 63%; aspirin, 95.1% versus 95.8%; β-blocker, 83.8% versus 88.6%; statin, 71.1% versus 73.4%; and thienopyridine, 89.6% versus 88.2%. In the national registry of MI during 1994-2003,40 there was a steady increase in the use of adjunctive agents such as aspirin, β-blockers, ACE inhibitors, other antiplatelet agents, and antithrombins within the first 24 hours of STEMI (Fig. 13-1).
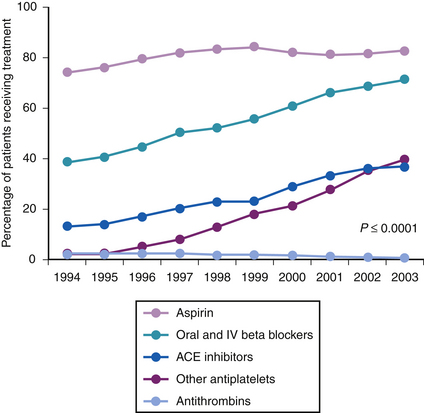
Figure 13-1 Medications received within the first 24 hours. ACE, angiotensin-converting enzyme.
(From Gibson CM: NRMI and current treatment patterns for ST-elevation myocardial infarction. Am Heart J 2004;148:S29-S33.)
Published American College of Cardiology and American Heart Association (ACC/AHA) practice guidelines for management of MI9–12 provide expert panel recommendations for initial reperfusion and concurrent or subsequent adjunctive therapies based on a ranking of the clinical evidence into class I, with benefit >>> risk (“should” be used); class II, with benefit >> or > risk (“reasonable” or “might be reasonable”); and class III, with risk > benefit (“should not” be used, “may be harmful”). There are clinical scenarios, however, in which deviations from these guidelines are considered appropriate.11,12 Ultimately, therapy should be tailored to the individual patient profile.
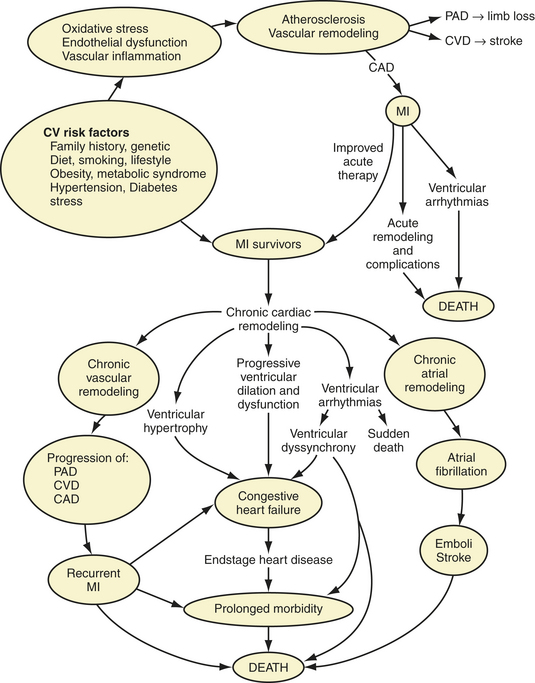
Consistent with the updated guidelines,10–12 unstable angina/NSTEMI12 is addressed separately in Chapters 8 and 16, whereas STEMI10,11 is addressed here. It is useful to time phases of adjunctive therapy according to temporal changes after MI, as follows: a very early or acute phase, in the first 12 to 24 hours; a subacute phase during healing, over 1 week to between 6 weeks and 6 months depending on infarct size and other factors; and a posthealing phase, after 1.5 to 12 months.19,23 Survivors of STEMI represent a special group of patients at double jeopardy for increased morbidity and mortality, and they stand to benefit from adjunctive therapies and comprehensive secondary prevention (Fig. 13-2).41
Practice pattern studies reveal suboptimal use of medications and invasive strategies in elderly MI patients (STEMI or NSTEMI) and lack of evidence-based data.27,28 Contraindications to reperfusion increase with age, and eligible elderly patients with STEMI are more likely to receive conservative than aggressive reperfusion therapy.28 Most first MIs occur in patients older than 65 years; the average age for a first MI is 66 in men and 70 in women.42 Adjunctive therapy plays an important role in elderly patients, but close attention to dosing and complications is needed pending more data. Results of studies on novel adjunctive therapies during the healing phase after reperfused STEMI in elderly patients are awaited.
Aspirin
Low-dose aspirin (acetylsalicylic acid) should be prescribed as adjunctive therapy to all acute MI patients who can tolerate it, including patients with STEMI, NSTEMI, and unstable angina. Platelets are important in thrombus formation after plaque rupture.29,43 Early thrombus is composed of a white clot consisting of aggregated platelets and a red clot consisting of fibrin and erythrocytes. Platelets are activated by fibrinolysis, and platelet-rich clots are more resistant to fibrinolysis than fibrin and erythrocyte thrombi. Aspirin is an effective antiplatelet agent that inhibits platelet aggregation.
Mode of Action
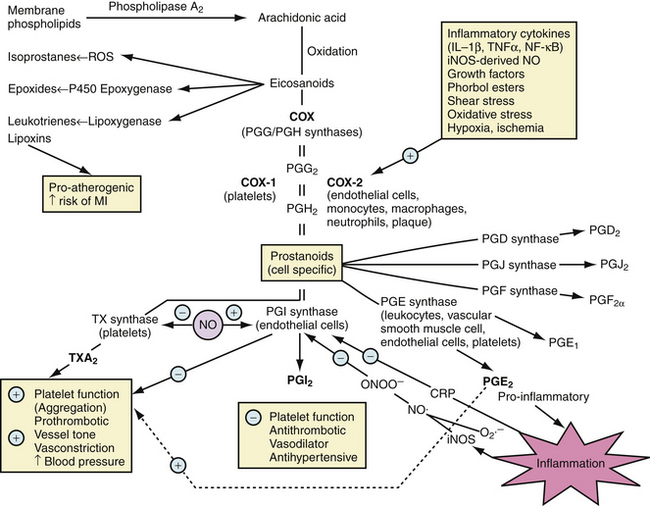
Taken orally, aspirin is rapidly absorbed in the stomach and small intestine. Aspirin is the only nonsteroidal anti-inflammatory drug (NSAID) to react covalently with the cyclooxygenase (COX) channel of prostaglandin (PG) G/H synthase-1 (now referred to as COX-1 and COX-2) through selective acetylation of serine residues Ser529 in human COX-144 and Ser516 in human COX-2.45 Constitutive COX-1 promotes platelet aggregation, thrombosis, and vasoconstriction, and protects gastrointestinal mucosa (Fig. 13-3).46 In contrast, inducible COX-2 is proinflammatory via PGE2 and antithrombotic and vasodilatory via PGI2 (prostacyclin) (see Fig. 13-3).46
Only a low dose is required to achieve irreversible acetylation of COX-1 in platelets and to block thromboxane A2 (TXA2) production and spare PGI2 synthesis.47 Higher doses are not more effective and might not spare PGI2.47 The effect of aspirin begins within 30 to 40 minutes and lasts for the life of the platelet (7 to 10 days).48,49 Nearly complete blockade of TXA2 can be achieved after 7 to 10 days of a daily dosage of 162 mg or more of aspirin. Side effects with low-dose aspirin, especially buffered tablets, are minimal. The cost is also low.
Dose, Timing, and Benefits
The efficacy of low-dose aspirin in acute STEMI was established in the randomized ISIS-2 trial, which assessed the effects of a 1-hour intravenous infusion of streptokinase (1.5 million U) or oral aspirin (160 mg) or both for 1 month in patients with acute MI presenting within 24 hours of the onset of symptoms.3 By 5 weeks, aspirin reduced nonfatal reinfarction by 50%, nonfatal stroke by 46%, total cardiovascular mortality by 23% (absolute risk reduction of 2.4%), and the risk of any vascular event by 23%. Reduction of cardiovascular mortality was enhanced by the combination of antiplatelet and fibrinolytic therapy, and cardiovascular mortality was decreased by 25% with streptokinase alone and by 42% with streptokinase and aspirin combined (absolute risk reduction of 5.2%), indicating that low-dose aspirin alone was as effective as streptokinase, and the combination produced synergism. Aspirin therapy might reduce the rate of reocclusion.3 Patients taking aspirin had fewer cardiac arrests, but slightly more minor bleeding. Aspirin did not increase the risk of cardiac rupture or bleeding requiring transfusion.
ISIS-2 was not only the largest aspirin trial in STEMI patients, but also the trial with the most robust evidence that aspirin reduces mortality in STEMI patients.3 Mortality reduction was similar in patients treated within 4 hours (by 25%), between 5 and 12 hours (by 21%), and between 13 and 24 hours (by 21%). A subsequent meta-analysis of MI trials using aspirin and the thrombolytics streptokinase and alteplase showed that aspirin reduces coronary reocclusion and recurrent ischemic events.50
On presentation, STEMI patients should be started on 162 to 325 mg of aspirin followed by 81 to 162 mg daily indefinitely (Table 13-1).10–1251 Non–enteric-coated aspirin should be used initially and chewed to ensure rapid buccal absorption.52,53 Long-term aspirin therapy for secondary and primary prevention has tended to use higher doses,54 although a low dose might be equally effective.51 A meta-analysis of small randomized trials of antiplatelet agents (aspirin, dipyridamole, or sulfinpyrazone, alone or in combination) for secondary prevention in survivors of previous cardiovascular events including acute MI indicated reduction in the risk of nonfatal acute MI by 32%, nonfatal stroke by 27%, total cardiovascular death by 15%, and vascular event by 25%. Aspirin alone was as effective as aspirin combined with another antiplatelet agent.55 A lower dose (300 mg daily) was as effective as higher doses (1000 to 1500 mg daily) for secondary prevention. A large U.S. trial on primary prevention showed that a low dose of aspirin (325 mg on alternate days) was highly effective and decreased the risk of acute MI by 44%.56 The resulting recommendation was that 160 mg of aspirin daily be given immediately after acute MI and be considered for primary prevention.
Table 13–1 Dosing for Oral Antiplatelet Therapy
| Drug | Loading Dose (mg) | Maintenance |
|---|---|---|
| Aspirin | 162-325 | 75-81 mg or 162 mg daily |
| Clopidogrel | 300-600∗ | 75 mg daily |
| Prasugrel | 60 | 10 mg daily |
| Ticlopidine | 500 | 250 mg twice daily |
Note: Drugs are listed alphabetically and not in order of preference.
∗ Depending on interventionalist’s preference.
In STEMI patients who have received stents for reperfusion, a higher dose regimen is recommended. Patients with bare metal stents should be given 325 mg of aspirin daily for 1 month; patients with drug-eluting stents should be given 325 mg aspirin daily for 3 to 6 months.57 In patients with unstable angina or NSTEMI, aspirin trials show mortality benefit independent of dose, initial timing, and duration of follow-up,58–61 and the 2007 ACC/AHA guideline panel recommended decreasing the poststent aspirin dose from 325 mg to between 162 to 325 mg daily.12 Aspirin does not prevent restenosis, and its benefits are attributed to prevention of in-stent thrombosis and secondary prevention.62 Based on evidence that lower doses of 75 to 150 mg are effective for secondary prevention,51,63 a daily dose of 75 to 162 mg indefinitely is recommended for secondary prophylaxis in patients without stents,57 and a similar dose is recommended after finishing the higher dose schedule in patients with stents.57
The dose range in aspirin recommendations is due to the lack of head-to-head trials comparing the low doses and high doses in patients with acute MI. Although there have been no randomized controlled trials on differing lengths of therapy, STEMI, unstable angina/NSTEMI, and chronic angina guidelines all state that aspirin for secondary prevention should be continued indefinitely.10–1264 Evidence for this recommendation comes from the Antiplatelet Trialists’ Collaboration review of 145 randomized controlled trials with aspirin over the long-term in patients with known coronary artery disease, prior stroke, or transient ischemic attack.65 There was statistically significant benefit for 2 years, although there was no statistically significant benefit in the third year. There was a 22% reduction in the composite end point of death, nonfatal recurrent infarction, and nonfatal stroke in patients with acute STEMI, patients at risk for STEMI, and patients with documented prior STEMI, with superior benefit in patients with the highest baseline risk.65
Adverse Effects
Aspirin can cause gastrointestinal bleeding and intracranial hemorrhage. Long-term or high-dose aspirin and other NSAIDs are associated with increased risk of gastrointestinal bleeding.66 Although several studies did not find increased bleeding risk from aspirin dosages of 75 to 325 mg daily,51,65,67–69 subset analyses from the nonrandomized CURE and BRAVO trials showed increased risk of bleeding from the low-dose to mid-dose range.68,69 Although the Antithrombotic Trialists’ Collaboration found no difference in bleeding between aspirin groups given less than 75 mg versus greater than 75 mg daily, there was still a small statistically nonsignificant increase in the risk of bleeding.51 A meta-analysis of aspirin dosing in cardiovascular prevention and side effects revealed no difference in bleeding risk with daily dose ranging from medium (75 to 162 mg) to low (<75 mg).67 Collective evidence supports low-dose aspirin (75 to 81 mg) for long-term use.70
Patients unable to tolerate oral medications because of severe nausea can be given a 300-mg aspirin suppository.61 An absolute contraindication to aspirin is evidence of hypersensitivity to salicylates61; most such patients manifest the Samter’s triad (sensitivity to salicylates, asthma, and nasal polyps).12 Patients with true allergy to aspirin can be given the thienopyridine clopidogrel or ticlopidine.10–12 Aspirin is also contraindicated in patients with bleeding conditions (e.g., retinal hemorrhage, active peptic ulcer, other serious gastrointestinal or urogenital bleeding, hemophilia, and untreated severe hypertension).12 In patients with prior gastrointestinal bleeding owing to ulcer after long-term use of low-dose aspirin and evidence of healed ulcer and eradication of Helicobacter pylori, 100 mg daily of aspirin plus the proton pump inhibitor lansoprazole for 12 months resulted in marked reduction of recurrent bleeding.71 In another study of patients with ulcer bleeding during aspirin therapy and evidence of healed ulcer and H. pylori eradication, comparison of clopidogrel plus the proton pump inhibitor esomeprazole versus low-dose aspirin (75 mg daily) plus a proton pump inhibitor showed the aspirin plus proton pump inhibitor combination to be superior in preventing recurrent ulcer bleeding.72
Based on these results,71,72 and evidence of the large benefit of aspirin after MI,51 aspirin combined with a proton pump inhibitor should be continued if possible, unless the bleeding is life-threatening or cannot be controlled otherwise. It is reasonable to use acetaminophen initially in patients at risk for gastrointestinal bleeding for pain control.66 If use of acetaminophen is impossible, it may be reasonable to use a selective COX-2 inhibitor in the short-term pending more long-term clinical data.66
The use of selective COX-2 inhibitors (COXIBs) and nonselective NSAIDs has been reviewed elsewhere.46,66,73–75 COXIBs in all dosages and nonselective NSAIDs in high doses increase mortality in patients with previous MI and should be avoided.73 Adverse effects of nonselective NSAIDs are attributed to loss of gastrointestinal protection and hemostasis via COX-1 and loss of anti-inflammatory activity via COX-2.46 COXIB-induced reduction of PGI2 and unchecked COX-1 activity result in continued TXA2 production and increased risk of thrombosis, which may be harmful during acute MI.46 COXIB-induced anti-inflammatory effects may be beneficial for progression of atherosclerosis, but harmful during infarct healing.46 Suppression of inflammation by COX inhibitors can impair infarct healing after STEMI and lead to infarct thinning, adverse left ventricular remodeling, aneurysm, decreased resistance to rupture, and cardiac rupture,46,76–80 suggesting the need for caution.
The U.S. Food and Drug Administration (FDA) issued a warning on the concomitant use of aspirin and the NSAID ibuprofen.75 Ibuprofen (but not rofecoxib, acetaminophen, or diclofenac) interferes with aspirin-induced acetylation of COX-1.71 The FDA also warned that COXIBs increase cardiovascular risk; rofecoxib (Vioxx) was withdrawn, although celecoxib and valdecoxib remain on the market.71 STEMI patients taking ibuprofen and requiring aspirin either should switch to another NSAID or should take ibuprofen 30 minutes after taking an immediate-release aspirin tablet or 8 hours before taking aspirin.75 Data on the concomitant use of enteric-coated aspirin and ibuprofen are lacking.75 One study showed, however, that the antiplatelet effect of enteric-coated low-dose aspirin was attenuated by ibuprofen (400 mg daily) given 2, 7, and 12 hours after aspirin.71
If COXIBs are used for pain control, they should be given “at the lowest possible dose and for the shortest time necessary.”71 In the 2007 guideline update, morphine is recommended for pain control in patients with acute STEMI whether reperfused or not (class I, evidence level C).11 On the basis of evidence that patients taking NSAIDs within the week before MI have an increased risk of death, hypertension, reinfarction, heart failure, myocardial rupture or shock,66,73,81–83 NSAIDs and COXIBs should be stopped in patients who develop acute STEMI (class I, evidence level C), and should not be given to them during hospitalization (class III, evidence level C).11,66 Data from a substudy of the ExTRACT TIMI-25 trial showed that STEMI patients taking NSAIDs within 7 days of enrollment were at increased risk of death, reinfarction, heart failure, or shock.83 A stepped-care approach has been recommended and is useful.66
The role of other antiplatelet agents, such as prostacyclin analogues, thromboxane antagonists, phosphodiesterase inhibitors, serotonin receptor antagonists, fibrinogen receptor antagonists, monoclonal antibodies directed against glycoprotein (GP) IIb/IIIa, von Willebrand factor inhibitors, thienopyridines, thrombin inhibitors, and nitrates, in treating and preventing acute MI and other acute coronary syndromes continues to be evaluated.84
Although aspirin is an effective antiplatelet drug, five points warrant mention. (1) Aspirin does not inhibit platelet degranulation in response to various stimuli or platelet adhesion to damaged endothelium.85 (2) Aspirin resistance develops in 40% of patients, mostly women and elderly patients.86,87 (3) Patients with PGIA2 polymorphism for GP IIb/IIIa show an enhanced platelet aggregation response to aspirin.88 (4) Aspirin has nonplatelet effects, such as inhibition of interleukin-6 synthesis in leukocytes89 and inhibition of endothelial nitric oxide inhibitors.90 (5) Low-dose aspirin inhibits platelet and vascular COX activity.91
Pericarditis is common after STEMI.79 Its timing coincides with the subacute phase during healing.19,23 Successful early reperfusion attenuates transmural extension and explains why Dressler’s syndrome is rare in the reperfusion era.92 Although NSAIDs, such as ibuprofen and indomethacin, and corticosteroids have been effective for pericarditis, they can cause infarct expansion and thinning and cardiac rupture,76–79,93–95 and should be avoided or used as a last resort. High-dose aspirin (650 mg every 4 to 6 hours) may be used for pain control.10,96,97 Alternatively, colchicine98–100 or acetaminophen10 may be used. Short-term corticosteroids and NSAIDs may be used with extreme caution.10,66,83 Ibuprofen should not be used because it attenuates the antiplatelet effect of aspirin71 and causes infarct thinning.76–7997 Misoprostol may be added for gastric and renal protection when NSAIDs are used.101,102 Data on the optimal management of pericarditis occurring in STEMI patients after reperfusion are awaited.
Summary of ACC/AHA Task Force Recommendations for Aspirin in ST Segment Elevation Myocardial Infarction
The ACC/AHA Task Force recommendations for aspirin in STEMI are summarized as follows.10,11
Thienopyridines
The thienopyridines, such as clopidogrel and ticlopidine, are antiplatelet drugs and are useful as adjunctive therapy after MI. The rationale is that, despite COX inhibition by aspirin, platelet activation can continue through TXA2-independent pathways leading to platelet aggregation and thrombin formation. Thienopyridines are recommended by the ACC/AHA management guidelines for STEMI and unstable angina/NSTEMI.10–12 Clopidogrel is preferred over ticlopidine because it has fewer side effects.103,104
Mode of Action
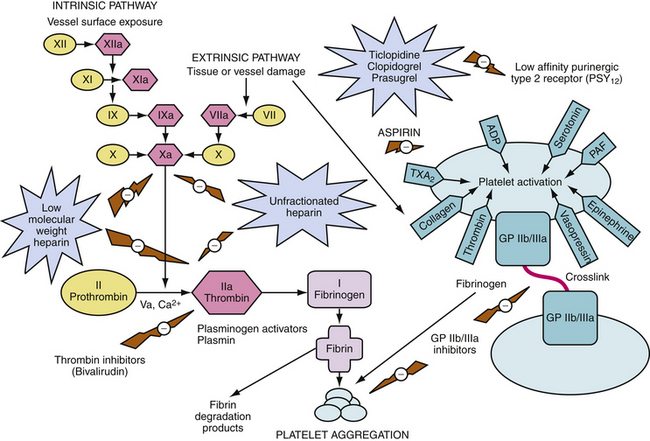
Thienopyridines block binding of adenosine 5′-diphosphate (ADP) to the low-affinity type 2 purinergic (P2Y12) receptor, inhibiting the binding of fibrinogen to the GP IIb/IIIa complex and preventing its activation, and resulting in inhibition of platelet aggregation (Fig. 13-4).105 Clopidogrel and ticlopidine do not affect the second type of high-affinity purinergic (P2Y1) receptor and do not affect COX. They have a more potent antiplatelet effect than aspirin. Both agents can be given orally, preferably after meals for maximal bioavailability. They are prodrugs and are activated in the liver and excreted by the kidneys.
Clopidogrel and ticlopidine have similar efficacy as aspirin for long-term prevention106,107 and may be substituted in patients with aspirin allergy or intolerance.12 In contrast to aspirin, thienopyridines produce significant inhibition after 2 to 3 days, and may take 4 to 7 days to achieve their full effect.105 Their platelet-inhibiting effects are long-lasting and persist for 7 to 10 days after therapy is stopped105; this corresponds to the life span of the platelet.
Dosing, Timing, and Benefits
Pending more data on the optimal loading dose of clopidogrel, 300 mg is used on the first day and followed by 75 mg daily in STEMI patients undergoing PCI and in unstable angina/NSTEMI patients being managed conservatively or undergoing PCI.10,12 A dose of 75 mg of clopidogrel is used in patients with aspirin allergy or intolerance.10,12 Clopidogrel should not be administered for 5 to 7 days before coronary artery bypass graft (CABG) surgery10,12,108 because of increased risk of bleeding with clopidogrel plus aspirin.108–110 Clopidogrel doses of 600 to 900 mg have been studied, and small to moderate size trials have compared 600 mg with 300 mg.12,111–113 Ticlopidine (loading dose 500 mg; maintenance dose 250 mg twice daily) is used in patients with allergy to clopidogrel.
Pending more data on optimal timing of clopidogrel in PCI, and based on data from PCI-CURE,114 the ACC/AHA guidelines recommend initiating therapy even if a conservative strategy is planned.10 The CURE trial showed benefit with clopidogrel started on hospital admission despite the need for cardiac surgery in some patients.115 Clopidogrel also benefited patients with low 0-2 Thrombolysis In Myocardial Infarction (TIMI) risk scores, although the largest benefit in terms of the primary end point of MI, stroke, or death occurred in the highest risk patients.116 Clopidogrel should no longer be considered only for patients undergoing PCI. At hospitals that have rapid access to the cardiac catheterization laboratory, clopidogrel is withheld until there is confirmation of the coronary anatomy and that the patient needs PCI rather than CABG surgery. The loading dose of clopidogrel (300 to 600 mg) is given on the catheterization table just before PCI.12 This approach is acceptable because of the increased risk of bleeding if the patient were to require urgent CABG surgery.
Based on findings of the PCI-CLARITY study in CLARITY-TIMI 28,117 clopidogrel should be started early (i.e., pretreatment) in cases of STEMI where angiography is delayed by 2 to 8 days because it results in a better outcome without increased risk of major or minor bleeding than when it is given just before angiography. In the CLARITY-TIMI 28 study,117 the addition of clopidogrel to background aspirin therapy in STEMI patients reduced the risk of clinical events (i.e., death, reinfarction, stroke). Because the patients enrolled in CLARITY117,118 were 75 years old or younger, guidance on whether STEMI patients older than 75 years should receive a loading dose of clopidogrel is awaited.
Data on the risks and benefits of performing early versus delayed CABG surgery in STEMI patients receiving clopidogrel are lacking. Evidence from the CRUSADE registry showed no difference, however, in death, reinfarction, or stroke in NSTEMI patients who did versus patients who did not receive clopidogrel and went for early CABG surgery, despite an increase in blood transfusion requirements associated with failure to delay surgery.12,108
Collective evidence indicates that all patients undergoing PCI should receive dual antiplatelet therapy with clopidogrel and aspirin.10,12,54,103,114,117–119 The length of clopidogrel therapy varies depending on the type of stent. For bare metal stents, aspirin at a dose of 162 to 325 mg daily plus clopidogrel at a dose of 75 mg daily for 1 month is necessary.12,54 Ideally, clopidogrel can be continued for 1 year if the risk for bleeding is low.54 Clopidogrel and aspirin may be continued for other reasons. There is controversy regarding the length of clopidogrel therapy with drug-eluting stents. The old PCI guidelines stated that clopidogrel should be given for at least 3 months for sirolimus-eluting stents and at least 6 months for paclitaxel-eluting stents, but ideally therapy should be continued for 1 year if the risk of bleeding is low.16
STEMI patients who have not undergone revascularization may also benefit from clopidogrel therapy. The COMMIT/CCS-2, in which half of the patients did not undergo revascularization, showed benefit of clopidogrel therapy in STEMI.120 Clopidogrel-treated patients in that study had a lower rate of the composite end point (death, reinfarction, stroke) and death (7.5% versus 8.1%), with no excess bleeding. In the CURE trial, NSTEMI patients who did not undergo stenting also benefited from clopidogrel therapy.109
The optimal length of clopidogrel therapy for secondary prevention in patients with acute coronary syndromes is not well defined.12,121,122 For a noninvasive strategy, the ACC/AHA guidelines for unstable angina/NSTEMI recommend clopidogrel for 1 month to 1 year after the event.12 The advice to extend therapy beyond 1 month is based primarily on data from the CURE trial.123 There is no evidence to support prolonging therapy beyond 1 year, however. Because the 2004 STEMI guidelines10 do not give advice for patients managed conservatively with thienopyridines, it is reasonable to follow the 2007 unstable angina/NSTEMI guidelines12 and recommend clopidogrel for at least 1 month and up to 1 year.
Adverse Effects
Ticlopidine therapy is associated with gastrointestinal upset, neutropenia, agranulocytosis, aplastic anemia, and rarely thrombotic thrombocytopenic purpura,103,104,124 requiring complete and differential blood counts every 2 weeks for 3 months. Clopidogrel therapy is associated with gastrointestinal upset and a low risk of thrombotic thrombocytopenic purpura but no neutropenia,106,125 Advantages of clopidogrel, such as once-daily dosing and no need for repeated laboratory monitoring, make it the preferred thienopyridine for the management of STEMI and NSTEMI and unstable angina.10,12,126,127 Typically, neutropenia from ticlopidine resolves within 1 to 3 weeks after stopping the drug and may rarely be fatal.12 Plasma exchange is required if thrombotic thrombocytopenic purpura develops.124–126 Ticlopidine is discouraged in patients with renal and hepatic dysfunction.
Although there is evidence that dual antiplatelet therapy with low-dose aspirin and clopidogrel reduces repeat cardiac events,109,114,128 the combination of aspirin and clopidogrel was not more effective than aspirin for primary prevention in high-risk patients in the CHARISMA trial.129 The primary end point in that study was MI, stroke, or cardiovascular death. There was a moderate benefit in the secondary end point of MI, stroke, cardiovascular death, or hospitalization for unstable angina, transient ischemic attack, or revascularization. Although there was no significant difference in severe bleeding, there was an increased risk of moderate bleeding in the group treated with aspirin plus clopidogrel. Moderate bleeding was defined as nonsevere bleeding requiring transfusion. The study showed that clopidogrel plus aspirin prevented 94 ischemic (secondary) end points at the cost of 93 moderate or severe bleeds.129 The patients with prior MI, ischemic stroke, and peripheral arterial disease derived significant benefit from dual therapy.130 A large international study in outpatients with atherothrombosis (coronary, cerebrovascular, and peripheral arterial) revealed high annual cardiovascular event rates and increased 1-year risk of events with multiple disease locations.131 Although evidence shows that patients may experience large interpatient responses to clopidogrel, with increased risk of thrombotic events if they have less than expected inhibition of platelet aggregation,132–134 a standardized monitoring technique and appropriate management still need to be determined.12
Summary of ACC/AHA Task Force Recommendations for Thienopyridines in ST Segment Elevation Myocardial Infarction
ACC/AHA Task Force recommendations for thienopyridines in STEMI are summarized as follows.10
Early Phase
Convalescent and Postdischarge Phases
β-Adrenergic Blockers
The rationale for β-blockade in acute MI is that it decreases myocardial oxygen demand (by decreasing heart rate, blood pressure, and contractility); improves the distribution of myocardial blood flow; and exerts desirable antiplatelet and antiarrhythmic effects. β-blockade has the potential for limiting infarct size, decreasing wall stress, and decreasing mortality. It is used in very early MI and over the long-term for secondary prevention after MI, regardless of the type (STEMI, NSTEMI, and unstable angina) or concomitant fibrinolytic therapy or primary PCI.10,12
Mode of Action
β-blockers compete with catecholamines for two β-adrenergic receptors; β1 receptors are primarily located in the myocardium and affect sinus node rate and atrioventricular (AV) node conduction velocity, and β2 receptors are primarily located in bronchial and vascular smooth muscle. β-blockers act on the cardiovascular system to slow heart rate, reduce blood pressure, and reduce cardiac contractility, resulting in decreased cardiac workload, myocardial oxygen consumption, and prolongation of diastolic filling time and coronary perfusion. Given acutely, β-blockers decrease infarct size in patients who do not receive reperfusion therapy, reduce the rate of reinfarction in patients who receive reperfusion therapy, and decrease the frequency of arrhythmias.10
Low doses of selective β-blockers block β1 more than β2 receptors, and may be preferable for patients with pulmonary and peripheral vascular disease. Some β-blockers have intrinsic sympathomimetic activity, resulting in activation of the β receptor, and less slowing of heart rate, less prolongation of AV node conduction time, and less depression of left ventricular function. Only nonselective β-blockers without intrinsic sympathomimetic activity were shown to reduce mortality after MI,138 and β-blockers with intrinsic sympathomimetic activity were unfavorable for secondary prevention.139 Two large post-MI trials of oral β-blockers in the era before reperfusion showed mortality benefit.140,141
Dosing, Timing, and Benefits
A meta-analysis of trials in the prethrombolytic era revealed a 7% reduction (not statistically significant) in mortality with oral β-blockade and 9% reduction (not statistically significant) in mortality with intravenous β-blockade.139 The β-blocker in the early studies was started within 24 hours, and the initial intravenous dose was 5 to 10 mg for propranolol,142 5 to 10 mg for atenolol,143 or 10 to 15 mg for metoprolol.144,145 In the largest trial,143 acute intravenous atenolol was followed by an oral dose of 100 mg for 1 week, and the mortality at 7 days was reduced by 14% (from 4.3% to 3.7%). In the MIAMI study,144 acute intravenous metoprolol was followed by 25 to 50 mg taken orally four times daily for 2 days and 100 mg twice daily thereafter, and the mortality at 15 days was decreased by 12% (from 4.9% to 4.3%). A substudy of MIAMI patients showed limitation of left ventricular remodeling and improved left ventricular function.145 A trial of β-blockade and concomitant thrombolytic therapy suggested a reduction in reinfarction rate.146 Several secondary prevention trials139,145,146 have shown improved survival and decreased reinfarction rate with long-term β-blocker therapy after acute MI.
In the ACC/AHA guidelines, intravenous β-blocker followed by oral dosing is recommended for acute MI patients without contraindications.9,10,12 In the absence of contraindications, β-blockers should be given to patients with hypertension, reflex tachycardia, rapid atrial fibrillation, or postinfarction angina. Agents with less intrinsic sympathomimetic activity and short duration of action are preferred.9 If tolerated, oral therapy should be continued for 2 years. Relative contraindications to β-blocker therapy include heart rate less than 60 beats/min, systolic blood pressure less than 100 mm Hg, moderate to severe left ventricular failure, signs of peripheral hypoperfusion, shock, PR interval greater than 0.24 second, second-degree or third-degree AV node block, active asthma, reactive airways disease, and MI induced by cocaine use.10,12
Intravenous β-blockers should be considered in all patients with STEMI who are not receiving fibrinolytic therapy or in patients who have ongoing resting chest pain.10,12 Patients who do not receive fibrinolytic therapy but receive intravenous β-blockers have been shown to have decreased infarct size and mortality.139,143,144 The ISIS-1143 and MIAMI144 trials showed a significant, sustained benefit in mortality after day 1 when patients were given either intravenous atenolol or intravenous metoprolol initially followed by oral dosing. Although patients who receive early fibrinolytic therapy may not be expected to benefit as much from intravenous β-blockers, the TIMI-II trial showed a benefit associated with the early use of intravenous β-blockers in terms of reduced recurrent ischemia and nonfatal reinfarction.144 Early administration of β-blockers within 2 hours of symptom onset reduced the composite end point of death or reinfarction.144
The routine use of intravenous β-blockers in patients receiving fibrinolytic therapy has been questioned on the basis of two randomized trials147,148 and a post-hoc analysis from the first GUSTO-1 trial showing no benefits.149 A review of early β-blockers in STEMI found no mortality benefit.150 Subsequently, the COMMIT/CCS-2 trial addressed the issue of early intravenous β-blockers in acute STEMI, randomly assigning 45,582 patients (93% STEMI or new left bundle branch block on electrocardiogram [ECG]), half of whom had fibrinolytic therapy, to three 5-mg intravenous metoprolol boluses followed by oral metoprolol extended-release, 200 mg daily for 28 days, or placebo; the trial found no difference in mortality.120 The β-blocker group in that study showed an increase in mortality, however, among patients who were hemodynamically unstable that was balanced by a decrease in mortality among patients who were hemodynamically stable.120 The β-blocker group also showed a decrease in ventricular fibrillation rate.120 For every 1000 metoprolol-treated patients, however, there were 5 fewer episodes of reinfarction and 5 fewer episodes of ventricular fibrillation (mostly on day 2 onward), but 11 more episodes of cardiogenic shock (mostly on days 0 to 1).120
The overall lack of benefit in COMMIT/CCS-2120 may have been due to the high dose of β-blocker given to patients who were hemodynamically unstable. Risk factors for cardiogenic shock from this study included older age, female sex, evidence of congestive heart failure (CHF), time delay in presentation, lower blood pressure, prior history of hypertension, and elevated heart rate.120 One key point from that study is that the administration of intravenous β-blockers should be targeted for specific indications, such as patients with ongoing chest pain at rest and no contraindications such as evidence of CHF. The 2007 update of the ACC/AHA guidelines recommends caution with the use of intravenous β-blockade, especially in patients who are at risk for cardiogenic shock.11 Data on the short-acting intravenous β-blocker esmolol in STEMI are awaited. The long-term use of oral β-blockers is recommended for secondary prevention in high-risk patients, after they have been stabilized, with gradual dose titration.151
The STEMI guidelines10 assign a class IIb recommendation for intravenous β-blockers, whereas the NSTEMI guidelines12 do not recommend early routine administration and support targeting use of β-blockers for specific indications. Both guidelines10,12 assign a class I recommendation for oral β-blockers. In patients receiving thrombolytics, there may be a potential benefit of early intravenous β-blockade in reducing the rate of intracranial hemorrhage.147,152 Data from a national registry on 60,532 patients treated with tissue-type plasminogen activator found that patients who received immediate intravenous β-blockers had lower rates of intracranial hemorrhage by 31%.152
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree