Fig. 9.1
Algorithm for the initial evaluation of the patient with suspected blunt abdominal trauma (FAST focused abdominal sonography for trauma, DPA diagnostic peritoneal aspirate, CT computed tomography, Hct hematocrit)
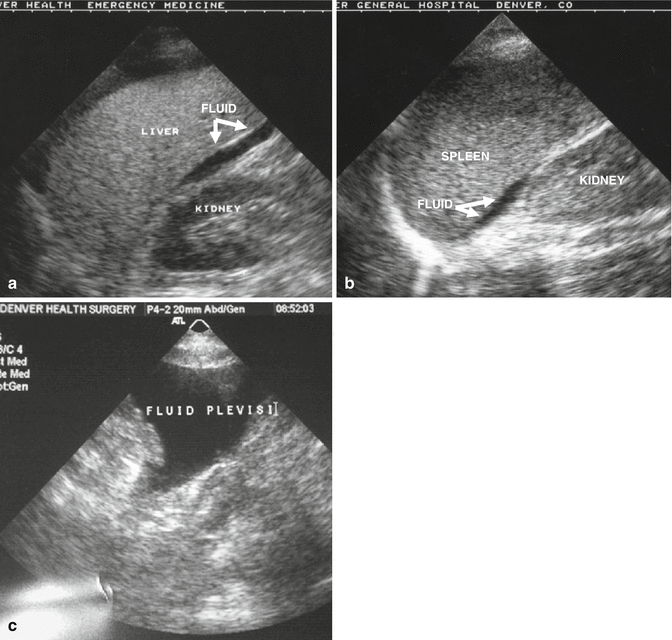
Fig. 9.2
FAST imaging detects intraabdominal hemorrhage. Hemorrhage is presumed when there is a fluid stripe visible between the right kidney and liver (a), left kidney and spleen (b), or in the pelvis (c)
9.2 Imaging for Abdominal Injuries
Based upon mechanism, location of injuries identified on physical examination, screening radiographs, and the patient’s overall condition, additional diagnostic studies may be indicated. Selective radiographs are done early in the patient’s ED evaluation. For patients with severe blunt trauma, chest and pelvic radiographs should be obtained. Historically, a lateral cervical spine radiograph was also obtained, hence the reference to the big three films, but currently patients preferentially undergo CT scanning of the spine rather than plain film radiography. Since its initial use in the early 1980s, CT scanning has become a routine part of the trauma evaluation for abdominal injury. With multi-slice helical scanning, the entire torso can be scanned in under 5 min. Patients with a positive FAST, who do not have immediate indications for laparotomy and are hemodynamically stable, undergo CT scan to quantify their injuries. Additionally, patients with persistent abdominal tenderness, significant abdominal wall trauma, distracting injuries, or altered mental status should undergo CT imaging. Although the majority of abdominal penetrating injuries that violate the peritoneum require laparotomy, the exception is penetrating trauma isolated to the right upper quadrant. In hemodynamically stable patients with the trajectory of penetrating trauma confined to the liver by CT scan, nonoperative observation is an option [7, 8].
CT scanning is excellent for identifying injuries of the solid organs (liver, spleen, kidney). If a diaphragmatic injury is not clearly identified on ED radiograph, CT scan can also be used to delineate these injuries, particularly with sagittal or coronal reconstructions (Fig. 9.3). Despite the increasing diagnostic accuracy of multi-slice CT scanners, CT still has limited sensitivity for identification of intestinal injuries. Bowel injury is suggested by findings of thickened bowel wall, “streaking” in the mesentery, free fluid without associated solid organ injury, or free intraperitoneal air [9].
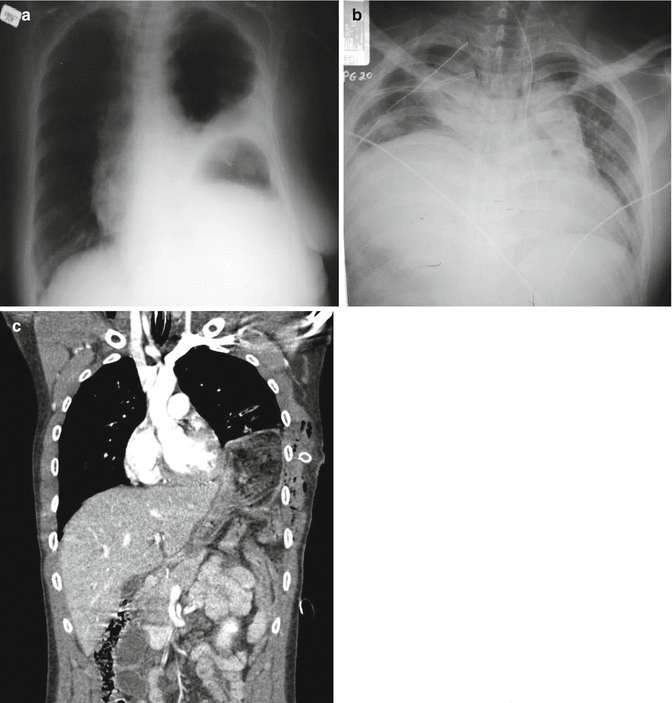

Fig. 9.3
Left diaphragm ruptures are evident with the gastric bubble located in the left hemithorax (a), while right-sided ruptures present with the appearance of an elevated hemidiaphragm (b). CT scanning may be used in questionable cases to better identify the injury (c)
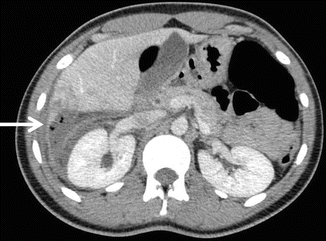
The American Association for the Surgery Trauma (AAST) developed a grading scale to provide a uniform definition of solid organ injuries based upon the magnitude of anatomic disruption (Table 9.1) [10]. Solid organ injury grading permits effective transfer of information between treating physicians, and predicts failure rates and complication rates of nonoperative management (NOM). In addition to grading the injury, specific findings that should be noted on CT scan include contrast extravasation (i.e., a “blush”), the amount of intraabdominal hemorrhage, and the presence of pseudoaneurysms (Fig. 9.4).
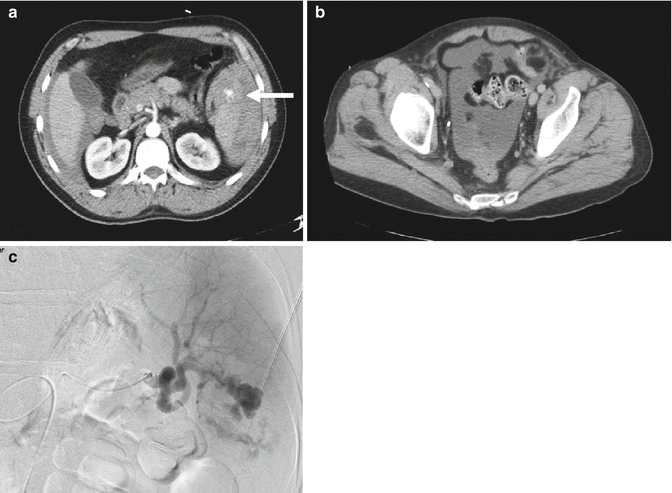
Table 9.1
AAST solid organ injury grading scales
Subcapsular hematoma | Laceration | |
|---|---|---|
Liver injury grade | ||
I | <10 % surface area | <1 cm in depth |
II | 10–50 % surface area | 1–3 cm |
III | >50 % or >10 cm | >3 cm |
IV | 25–75 % of a hepatic lobe | |
V | >75 % of a hepatic lobe | |
VI | Hepatic avulsion | |
Spleen injury grade | ||
I | <10 % surface area | <1 cm in depth |
II | 10–50 % surface area | 1–3 cm |
III | >50 % surface area | >3 cm |
IV | >25 % devascularization | Hilar injury |
V | Shattered spleen | |

Fig. 9.4
Findings on imaging that are associated with failure of NOM for splenic injuries: contrast extravasation or “blush” (arrow) (a), intraabdominal hemorrhage extending into the pelvis (b), and pseudoaneurysms (c)
9.3 Penetrating Injuries
The diagnostic approach differs between penetrating and blunt abdominal trauma. As a rule, minimal evaluation is required prior to laparotomy for gunshot or shotgun wounds that violate the peritoneal cavity, because over 90 % of patients have significant internal injuries. Anterior truncal GSWs between the fourth intercostal space and the pubic symphysis, whose trajectory by x-ray or entrance/exit wound indicates peritoneal penetration, should undergo operative exploration (Fig. 9.5). GSWs to the back or flank are more difficult to evaluate because of the retroperitoneal location of the injured abdominal organs. Triple-contrast CT scan can delineate the trajectory of the bullet and identify peritoneal violation or retroperitoneal entry, but may miss specific injuries [11]. Similarly, in obese patients if the GSW is thought to be tangential through the subcutaneous tissues, CT scan can delineate the tract and exclude peritoneal violation. Laparoscopy is another option to assess peritoneal penetration, and is followed by laparotomy to repair injuries if found. If in doubt, it is always safer to explore the abdomen than to equivocate, but a period of close observation in the patient with a reliable examination and hemodynamic stability may be considered.
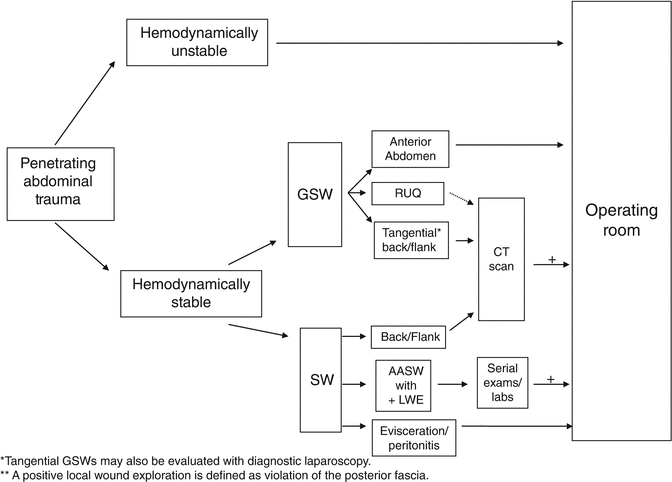

Fig. 9.5
Algorithm for the evaluation of penetrating abdominal injuries (GSW gunshot wound, SW stab wound, RUQ right upper quadrant, AASW anterior abdominal stab wound, LWE local wound exploration)
In contrast to GSWs, SWs that penetrate the peritoneal cavity are less likely to injure intraabdominal organs. Anterior abdominal SWs (from costal margin to inguinal ligament and bilateral mid-axillary lines) should be explored under local anesthesia in the ED to determine if the fascia has been violated. Injuries that do not penetrate the peritoneal cavity do not require further evaluation, and the patient is discharged from the ED. Patients with fascial penetration must be further evaluated for intraabdominal injury, as there is up to a 50 % chance of requiring laparotomy. The optimal diagnostic approach remains debated between serial examination, diagnostic peritoneal lavage (DPL), and CT scanning; the most recent evidence supports serial examination and laboratory evaluation [12].
Abdominal SWs of three body regions require a unique diagnostic approach: thoracoabdominal SWs, right upper quadrant SWs, and back/flank SWs. Occult injury to the diaphragm must be ruled out in patients with SWs to the lower chest. Diagnostic laparoscopy or DPL can be used to exclude diaphragmatic injury. Patients undergoing DPL evaluation have different laboratory value cut-offs than those traditional values formerly used for abdominal stab wounds (Table 9.2). A RBC count of more than 10,000/μL is considered positive, and an indication for laparotomy while patients with a DPL RBC count between 1,000/μL and 10,000/μL should undergo laparoscopy or thoracoscopy. A RBC count of less than 1,000/μL is considered negative; that is, the red cells are due to the procedure itself. Alternatively, laparoscopy may be preferred in those who cannot tolerate a DPL or those with evidence of a hemothorax or pneumothorax on chest radiograph. SWs to the flank and back should undergo triple-contrast CT to assess for retroperitoneal injuries of the colon, duodenum, and urinary tract [11] (Fig. 9.6).
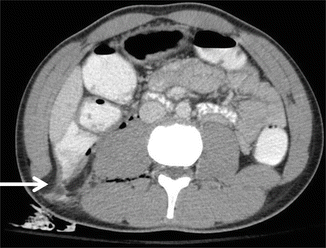
Table 9.2
A positive diagnostic peritoneal lavage following trauma is defined by specific laboratory values
Laboratory study | Positive value | |
|---|---|---|
AASW | TSW | |
White blood cell (WBC) | >500 cells/μL | >500 cells/μL |
Red blood cell (RBC) | >100,000 cells/μL | >10,000 cells/μL |
Amylase | >19 IU/L | >19 IU/L |
Alkaline phosphatase | >2 IU/L | >2 IU/L |
Bilirubin | >0.1 mg/dL | 0.1 mg/dL |

Fig. 9.6
Triple-contrast CT scan of the abdomen can delineate retroperitoneal injuries such as this colon injury (with contrast extravasation) (arrow) following a stab wound to the back
Although not universally embraced, selected patients with penetrating injuries to the right upper quadrant may be candidates for NOM [13–16]. Patients must have a CT scan that documents confinement of the injury to the liver (Fig. 9.7). Additionally, the patient must be hemodynamically stable, have a reliable physical examination without evidence of peritonitis (i.e., cannot have depressed mental status), and not require blood products. Patients should be admitted for serial examination and hemoglobin monitoring; any alteration should prompt laparotomy. Violation of the diaphragm has the risk of a biliopleural fistula. An alternative approach is to perform laparoscopy to confirm trajectory of the missile or knife, and to repair the diaphragm. In addition to avoiding the morbidity of a laparotomy, the success of NOM for penetrating trauma has resulted in decreased hospital stays, lower transfusion requirements, and diminished abdominal infection rates.
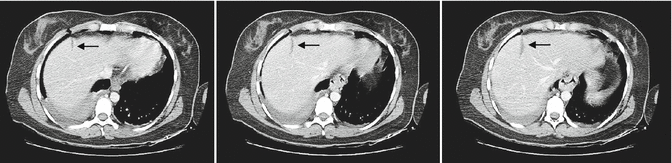

Fig. 9.7
Nonoperative management of penetrating abdominal trauma may be considered if the wound is isolated to the liver as documented by CT scan (arrow points to stab wound tract in liver)
9.4 Blunt Abdominal Trauma
9.4.1 Liver and Spleen
With the advent of CT scanning, NOM of solid organ injuries has replaced routine operative exploration. NOM of blunt solid organ injuries is appropriate in hemodynamically stable patients that do not have overt peritonitis or other indications for laparotomy. High-grade injuries, a large amount of hemoperitoneum, contrast extravasation, and pseudoaneurysms are not absolute contraindications for nonoperative management; however, these patients are at high risk for failure and are more likely to need angioembolization [17–21]. Likewise, there is not a patient age cutoff for the NOM of solid organ injuries. A multidisciplinary approach including angiography with selective angioembolization has improved NOM success rates as well as survival [19, 20, 22]. Over 80 % of patients with liver injuries may be managed nonoperatively.
Patients who require laparotomy for their liver injuries typically fail NOM in the first 24–48 h [17, 22]. Patients with persistent hemodynamic instability despite red cell transfusions of 4 units in 6 h or 6 units in 24 h should undergo laparotomy. Patients that develop peritonitis following admission should also undergo laparotomy with concern of a missed bowel injury. Of the minority (8 %) of patients that fail NOM, half require operation due to associated injuries (i.e., enteric or pancreatic injuries) while half undergo laparotomy for hepatic-related hemorrhage [17]. Predicting which patients will ultimately require laparotomy has yet to be accomplished. Perhaps not surprisingly, those patients who fail NOM have increasing rates of failure associated with increasing grades of hepatic injury. Series published to date report failure rates of 14 % in grade IV injuries and 23 % in grade V injuries [18]. The amount of hemoperitoneum appears to inversely correlate with successful management; patients with a large amount of hemoperitoneum (i.e., blood extending into the pelvis) are more likely to fail NOM.
An indication for angioembolization to address ongoing hepatic bleeding is transfusion of 4 units of RBCs in 6 h or 6 units of RBCs in 24 h in the hemodynamically stable patient. Hemodynamic instability, however, often requires laparotomy with perihepatic packing for hemostasis. Patients with contrast extravasation identified on CT scanning, indicating arterial hemorrhage, should also be considered as a candidate for hepatic angiography. Originally, evidence of extravasation was an indication for laparotomy; however, the advent of endovascular techniques has resulted in effective hemostasis in selected cases. Angioembolization is particularly helpful in hemodynamically stable patients with contrast pooling within the hepatic parenchyma [19]. Patients with contrast extravasation into the peritoneal cavity are more likely to require laparotomy [20], but cases of successful embolization have been reported [21].
Until the 1970s, splenectomy was considered mandatory for all splenic injuries. Recognition of the immune function of the spleen refocused efforts on splenic salvage in the 1980s [23, 24]. Following success in pediatric patients, NOM of splenic injuries was adopted in the adult population, and has become the prevailing strategy for blunt splenic trauma [25]. NOM of solid organ injuries is pursued in hemodynamically stable patients that do not have overt peritonitis or other indications for laparotomy [26–30]. Similar to liver injuries, there is not an age cutoff for patients for the NOM of splenic injuries [31, 32]. High-grade injuries, a large amount of hemoperitoneum, contrast extravasation, and pseudoaneurysms are not absolute contraindications for NOM; however, these patients are at high risk for failure [33–36]. The identification of contrast extravasation as a risk factor for failure of NOM led to liberal use of angioembolization in an attempt to avoid laparotomy. The true value of angioembolization in splenic salvage has not been rigorously evaluated. Patients with intraparenchymal splenic blushes who are otherwise asymptomatic may be considered for a period of observation rather than empiric angioembolization [37]; it is thought that the contained hemorrhage within the splenic capsule may result in tamponade of the bleeding (Fig. 9.8).

Fig. 9.8
Intraparenchymal splenic blush noted on initial CT scan (a, b) may resolve following a period of close observation (c) (arrow points to contrast extravasation)
It is clear, however, that 20–30 % of patients with splenic trauma deserves early splenectomy, and that failure of NOM often represents poor patient selection [38, 39]. In adults, indications for prompt laparotomy include initiation of blood transfusion within the first 12 h considered to be secondary to the splenic injury, or hemodynamic instability. In the pediatric population, blood transfusions up to half the patient’s blood volume are utilized prior to operative intervention. Following the first 12 postinjury hours, indications for laparotomy are not as black and white. Determination of the patient’s age, comorbidities, current physiology, degree of anemia, and associated injuries will determine the use of transfusion alone versus intervention with either embolization or operation. Unlike hepatic injuries, which rebleed in 24–48 h, delayed hemorrhage or rupture of the spleen can occur up to weeks following injury. Overall, nonoperative treatment obviates laparotomy in more than 90 % of cases.
9.4.2 Pancreatic Injuries
Pancreatic contusions, with or without associated ductal disruption, are difficult to diagnose in patients with blunt abdominal trauma [40]. Patients clearly at risk include those with significant mechanisms including high force, a seatbelt sign on physical examination, or a blow to the epigastrium [41]. The initial CT scan may show nonspecific stranding of pancreas. Associated fluid around the pancreas should prompt further invasive studies such as ERCP or MRCP to rule out a biliary or pancreatic duct injury. With a tentative diagnosis of a pancreatic contusion, one may consider following serial determinations of amylase/lipase; although these lab studies do not have a reliable sensitivity [42], increasing values over time combined with an alteration in clinical exam should prompt a repeat CT scan, duodenal C-loop study, DPL, or an ERCP depending upon the suspected lesion.
Historically, injuries to the pancreas were managed with operative intervention [43]. With the recent evolution of NOM for solid organ injuries, a nonresectional management schema has developed for select pancreatic injuries [44, 45]. Observation of pancreatic contusions, particularly those in the head of the pancreas that may involve ductal disruption, includes serial exams and monitoring of serum amylase. Patients with pancreatic injuries involving the major ducts, originally a strict indication for operative intervention, may be managed with ERCP and stenting in select patients; durability of this approach is currently under investigation [46].
9.4.3 Bowel Injuries
Diagnosing a hollow viscus injury is notoriously difficult [47], and even short delays in diagnoses result in increased morbidity [48, 49]. Findings suggestive of a bowel injury include thickening of the bowel wall, “streaking” in the mesentery, or free intraperitoneal air [9] (Fig. 9.9). If a patient’s initial CT scan of the abdomen shows free fluid without evidence of a solid organ injury to explain such fluid, evaluation for a bowel injury should be performed [50–52]. DPL should also be considered in a patient if there is increasing intraabdominal fluid on bedside ultrasound in patients with a solid organ injury but a stable hematocrit, and/or in patients with unexplained clinical deterioration. Particular attention should be paid to elevations in the DPL effluent of bilirubin, alkaline phosphatase, and amylase when pursuing a diagnosis of bowel injury, with specific laboratory values indicating need for laparotomy (Table 9.2) [53, 54]. A rectal injury may be life threatening in patients with pelvic fractures. While some patients have clear findings on physical examination, ranging from hematochezia to overt degloving of the perineum, others may have occult injuries that are missed on initial evaluation in the trauma bay. Flexible or rigid sigmoidoscopy should rule out blood within the canal, clear intestinal perforation, or ischemic mucosa [55].