484–406 BC
Adrenal and thyroid disorders are rarely the primary reason for admission to an ICU. However, critical illness can influence adrenal and thyroid function, and there is concern that this influence can have a negative impact on outcomes. This chapter describes the spectrum of adrenal and thyroid disorders that occur in critically ill patients, and how to detect and manage each of these disorders.
ADRENAL SUPPRESSION IN THE ICU
The adrenal gland plays a major role in the adaptive response to stress. The adrenal cortex releases glucocorticoids and mineralocorticoids that promote glucose availability and maintain extracellular volume, while the adrenal medulla releases catecholamines that support the circulation. Attenuation or loss of this adrenal response leads to hemodynamic instability, volume depletion, and defective energy metabolism (1,2). Adrenal insufficiency can remain silent until the adrenal gland is called on to respond to a physiologic stress. When this occurs, adrenal insufficiency becomes an occult catalyst that speeds the progression of acute, life-threatening conditions.
The activity of the adrenal glands is governed by the release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland, which, in turn, is governed by the production of corticotrophin-releasing hormone (CRH) in the hypothalamus (see Figure 50.1). Adrenal insufficiency can be the result of suppression at the hypothalamic-pituitary level, or primary suppression of adrenal gland activity.
Cortisol
Cortisol (hydrocortisone) is the major glucocorticoid released by the adrenal cortex. The daily production of cortisol in the normal (un-stressed) adult is 15–25 mg/day, and this can increase up to 350 mg/day during periods of maximum physiological stress (2).
PLASMA CORTISOL: About 90% of the cortisol in plasma is bound to corticosteroid-binding globulin (CBG) and albumin, while the remaining 10% is the free or biologically active form (1,2). The commercial assay for plasma cortisol measures both bound and unbound fractions; i.e., total cortisol. This assay can be misleading in acutely ill patients because plasma levels of CBG fall by as much as 50% during an acute illness (2). In one study of ICU patients with sepsis, total cortisol levels were diminished in 40% of the patients, while free cortisol levels were consistently elevated (4).
Critically Ill Patients
Adrenal insufficiency is common in critically ill patients. The overall prevalence is 10–20% (1), but rates as high as 60% have been reported in patients with severe sepsis and septic shock (3). The adrenal suppression in critically ill patients is often reversible, and is called critical illness-related corticosteroid insufficiency (CIRCI) (1). The mechanisms involved in CIRCI are complex, and are shown in Figure 50.1 (1–3,5). The systemic inflammatory response plays a major role in CIRCI. Suppression at the hypothalamic-pituitary level is particularly prominent, and is responsible for as many as 75% of the cases of adrenal suppression in patients with severe sepsis and septic shock (3).
Predisposing Conditions
As mentioned, severe sepsis and septic shock are the leading causes of adrenal suppression in critically ill patients. Other infection-related causes of adrenal suppression include HIV infection, systemic fungal infections, and meningococcemia (which can precipitate adrenal hemorrhage) (2,5).
Noninfectious sources of adrenal suppression in ICU patients include: (a) abrupt discontinuation of chronic steroid therapy, (b) adrenal hemorrhage from disseminated intravascular coagulation (DIC) or anticoagulant therapy, and (c) drugs that inhibit the synthesis of cortisol (e.g., etomidate and ketoconazole) or accelerate the metabolism of cortisol (e.g., phenytoin or rifampin) (2,5).
Clinical Manifestations
The principal manifestation of adrenal suppression in critically ill patients is hypotension that is refractory to volume resuscitation (1–3). The typical electrolyte abnormalities that accompany adrenal insufficiency (i.e., hyponatremia and hyperkalemia) are uncommon in the adrenal suppression associated with critical illness.
Diagnosis
Adrenal suppression should be suspected in any ICU patient with hypotension that does not respond to volume resuscitation. Unfortunately, the diagnosis of adrenal suppression in critically ill patients is steeped in uncertainty. (Note: The use of total cortisol levels to evaluate adrenal function is a major problem in critically ill patients because of the confounding influence of plasma proteins on total cortisol measurements, as mentioned previously.)
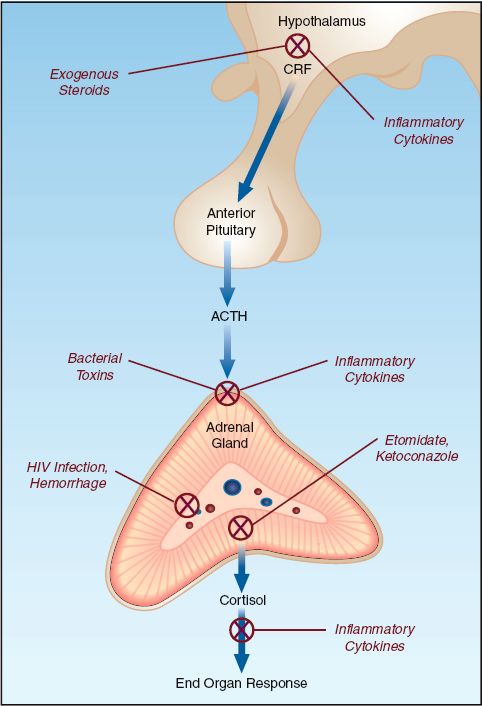
FIGURE 50.1 Mechanisms of adrenal suppression in ICU patients. CRF = cortico-trophin-releasing hormone; ACTH = adrenocorticotrophic hormone.
Rapid ACTH Stimulation Test
A popular (but often unnecessary) test of adrenal function in ICU patients is the rapid ACTH stimulation test, which can be performed at any time of the day or night. A blood sample is obtained for a baseline (random) plasma cortisol level, and the patient is given synthetic ACTH (Cosyntropin) intravenously in a dose of 250 ∝g. One hour after the ACTH injection, a second blood sample is obtained for a repeat plasma cortisol level. The interpretation of the test results is as follows (1,2):
The best predictor of adrenal suppression in critically ill patients is a random plasma cortisol level <10 ∝g/dL, or an increment in plasma cortisol of <9 ∝g/dL after the intravenous injection of synthetic ACTH (250 ∝g).
The favorite approach to evaluating adrenal function in the ICU is to rely on the random plasma cortisol level. A level that is ≥35 ∝g/dL is evidence of normal or adequate adrenal function, while a baseline cortisol level that is below 10 ∝g/dL is evidence of adrenal suppression. A rapid ACTH stimulation test can be performed when the random serum cortisol level is 10–34 ∝g/dL. However, a normal response to ACTH (i.e., an increment in serum cortisol of ≥9 ∝g/dL) does not eliminate the possibility of secondary adrenal suppression due to hypothalamic-pituitary dysfunction (which may be more common than suspected in ICU patients).
Septic Shock
In patients with septic shock, plasma cortisol levels are not necessary for identifying patients who might benefit from corticosteroid therapy. In these patients, a trial of intravenous hydrocortisone is recommended when hypotension is refractory to volume resuscitation (and requires a vasopressor drug).
Treatment
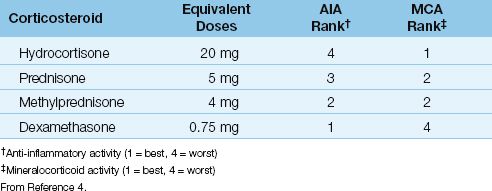
The treatment of critical illness-related adrenal suppression is intravenous hydrocortisone in a dose of 200–300 mg daily (i.e., 50 mg every 6 hours, or 100 mg every 8 hours) (1). The addition of a mineralocorticoid (i.e., fludrocortisone, 50 ∝g orally once daily) is considered optional (1), because hydrocortisone has excellent mineralocorticoid activity (see Table 50.1).
Hydrocortisone can be discontinued after satisfactory resolution of the underlying condition. In septic shock, hydrocortisone can be discontinued when vasopressor therapy is no longer necessary, and serum lactate levels have normalized. A gradual taper of the hydrocortisone dose (over at least a few days) is recommended to prevent a rebound increase in proinflammatory mediators (1).
Table 50.1 Corticosteroid Comparisons
EVALUATION OF THYROID FUNCTION
Laboratory tests of thyroid function can be abnormal in up to 90% of critically ill patients (6). In most cases, the abnormality is a consequence of non-thyroidal illness, and is not a sign of pathologic thyroid disease (6,7). This section describes the laboratory evaluation of thyroid function, and explains how to identify non-thyroidal illness as a cause of abnormal thyroid function tests.
Thyroxine (T4) and Triiodothyronine (T3)
Thyroxine (T4) is the principal hormone secreted by the thyroid gland, but the active form is triiodothyronine (T3), which is formed by deiodination of thyroxine in extrathyroidal tissues. Both T3 and T4 are extensively (>99%) bound to plasma proteins (especially thyroxine-binding globulin), and less than 1% of either hormone is present in the free, or biologically active, form (8). Because of the potential for alterations in plasma proteins and protein binding in acute illness, free T4 and T3 levels are more reliable for assessing thyroid function in ICU patients. Free T3 levels are not routinely available, so free T4 levels are used to evaluate thyroid function in acutely ill patients.
Thyroid-Stimulating Hormone (TSH)
The plasma level of thyroid-stimulating hormone (TSH) is considered the most reliable test of thyroid function, and is useful for identifying non-thyroidal illness, and for distinguishing between primary and secondary thyroid disorders. Plasma TSH levels have a diurnal variation, with the lowest values in late afternoon, and the highest values around the hour of sleep. TSH levels can vary by as much as 40% over a 24-hour period (9), and this diurnal variation must be considered when interpreting changes in plasma TSH levels. The reference range for serum TSH is 0.3–4.5 mU/dL (10).
Primary vs. Secondary Thyroid Disorders
Because of the negative feedback exerted by the thyroid hormones on TSH secretion, plasma TSH levels can distinguish primary from secondary thyroid disorders. For example, in patients with hypothyroidism, an elevated plasma TSH level is evidence of primary hypothyroidism, while a reduced TSH level is evidence of secondary hypothyroidism due to hypothalamic–pituitary dysfunction.
Non-Thyroidal Illness
Plasma TSH levels are normal in a majority of instances where abnormal thyroid function tests are the result non-thyroidal illness (6). However, plasma TSH levels can be depressed in 30%, and elevated in 10%, of these patients (6). TSH secretion can be depressed by sepsis, corticosteroids, and dopamine infusions (11), and these factors must be considered when interpreting plasma TSH levels.
Patterns of Abnormal Thyroid Function Tests
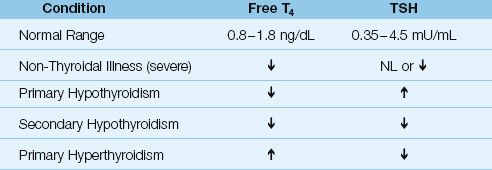
The interpretation of free T4 and TSH levels is shown in Table 50.2.
Table 50.2 Patterns of Abnormal Thyroid Function Tests
Non-Thyroidal Illness
Acute, non-thyroidal illness is associated with low plasma levels of free T3, as a result of impaired conversion of T4 to T3 in non-thyroidal tissue (6). With increasing severity of illness, both free T3 and free T4 levels are depressed, which is the pattern reported in 30–50% of ICU patients (6,7). As mentioned earlier, plasma TSH levels are normal in a majority of patients with non-thyroidal illness, but TSH levels can be depressed in selected conditions (e.g., sepsis, corticosteroid therapy, or dopamine infusions).
Thyroid Disorders
Primary thyroid disorders are characterized by reciprocal changes in free T4 and TSH levels, while in secondary thyroid disorders (due to hypothalamic-pituitary dysfunction), free T4 and TSH levels change in the same direction.
THYROTOXICOSIS
Thyrotoxicosis is almost always the result of primary hyperthyroidism. Notable causes include autoimmune thyroiditis, and chronic therapy with amiodarone (12).
Clinical Manifestations
The principal manifestations of thyrotoxicosis are agitation, tachycardias (including atrial fibrillation), and fine tremors. Elderly patients with hyperthyroidism can be lethargic rather than agitated; this condition is called apathetic thyrotoxicosis. The combination of lethargy and atrial fibrillation is a frequently cited presentation for apathetic thyrotoxicosis in the elderly (13).
Thyroid Storm
An uncommon but severe form of hyperthyroidism known as thyroid storm can be precipitated by acute illness or surgery. This condition is characterized by hyperpyrexia (body temperatures can exceed 104°F), severe agitation or delirium, and severe tachycardia with high-output heart failure. Advanced cases are associated with obtundation or coma, generalized seizures, and hemodynamic instability. If overlooked and left untreated, the outcome is uniformly fatal (12).
Diagnosis
The plasma TSH assay is the most sensitive and specific diagnostic test for hyperthyroidism, and is recommended as the initial screening test for suspected hyperthyroidism (12). TSH levels are <0.01 mU/dL in mild cases of hyperthyroidism, and TSH levels are undetectable in most cases of overt thyrotoxicosis (12). A normal TSH level (0.3–0.45 mmU/dL) excludes the diagnosis of hyperthyroidism (12).
Management
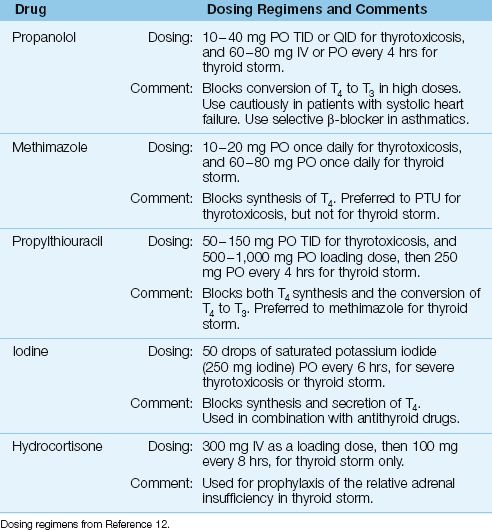
Table 50.3 includes the drugs and dosing regimens used for the treatment of thyrotoxicosis and thyroid storm.
ß-Receptor Antagonists
Treatment with β-Receptor antagonists relieves the tachycardia, agitation, and fine tremors in thyrotoxicosis. Propranolol has been the most widely used β-Receptor antagonist in hyperthyroidism (see Table 50.3 for the dosing recommendations), but it is a non-selective β-Receptor antagonist, which is not optimal for patients with asthma. More selective β-Receptor antagonists like metoprolol (25–50 mg PO every 4 hours) or esmalol (see Table 15.1 for dosing recommendations) can be used for thyrotoxicosis. However, propranolol remains the drug of choice for treatment of thyroid storm (12).
Antithyroid Drugs
Two drugs are used to suppress thyroxine production: methimazole and propylthiouracil (PTU). Both are given orally. Methimazole is preferred for the treatment of thyrotoxicosis, while PTU is favored for the treatment of thyroid storm (12). Uncommon but serious side effects include cholestatic jaundice for methimazole, and fulminant hepatic necrosis, plus agranulocytosis, for PTU (12). (See Table 50.3 for the dosing regimens for each drug).
Table 50.3 Drug Therapy for Thyrotoxicosis and Thyroid Storm
Inorganic Iodine
In severe cases of hyperthyroidism, iodine (which blocks the synthesis and release of T4) can be added to antithyroid drug therapy. The iodine is given orally as a saturated potassium iodide solution (Lugol’s solution). In patients with an iodine allergy, lithium (300 mg orally every 8 hours) can be used as a substitute (14).
Special Concerns in Thyroid Storm
In addition to the above measures, the management of thyroid storm often requires aggressive volume resuscitation to replace fluid losses from vomiting, diarrhea, and heightened insensible fluid loss. Thyroid storm can accelerate glucocorticoid metabolism and create a relative adrenal insufficiency, and prophylactic therapy with intravenous hydrocortisone (300 mg IV as a loading dose, followed by 100 mg IV every 8 hours) is recommended for thyroid storm (12).
HYPOTHYROIDISM
Symptomatic hypothyroidism is uncommon, with a prevalence of only 0.3% in the general population (15). Most cases are the result of chronic autoimmune thyroiditis (Hashimoto’s thyroiditis). Other causes include radioiodine, thyroidectomy, or hypothalamic-pituitary dysfunction from tumors and hemorrhagic necrosis (Sheehan’s syndrome), and drugs (lithium, amiodarone).
Clinical Manifestations
The clinical manifestations of hypothyroidism are often subtle, and include dry skin, fatigue, muscle cramps, and constipation. Contrary to popular perception, obesity is not a consequence of hypothyroidism (15). More advanced cases of hypothyroidism can be accompanied by hypo-natremia and a skeletal muscle myopathy, with elevations in muscle enzymes (creatine phosphokinase and aldolase), and an increase in the serum creatinine (from creatine released by skeletal muscle) that is not caused by renal dysfunction (16).
Effusions
The most common cardiovascular manifestation of hypothyroidism is pericardial effusion, which is the most common cause of an enlarged cardiac silhouette in patients with hypothyroidism (17). These effusions usually accumulate slowly and do not cause cardiac compromise. Pleural effusions are also common in hypothyroidism. The pleural and pericardial effusions in hypothyroidism are due to an increase in capillary permeability, and are exudative in quality.
Myxedema
Advanced cases of hypothyroidism are accompanied by an edematous appearance known as myxedema. This condition is mistaken for edema, but is caused by the intradermal accumulation of proteins (18). Myx-edema is also associated with hypothermia and depressed consciousness. The latter condition is called myxedema coma, even though unresponsiveness is uncommon (18).
Diagnosis
Serum T3 levels can be normal in hypothyroidism, but free T4 levels are always reduced (15). Serum TSH levels are elevated (often above 10 mU/dL) in primary hypothyroidism, and are depressed in hypothyroidism due to hypothalamic-pituitary dysfunction.
Thyroid Replacement Therapy
The treatment for mild to moderate hypothyroidism is levothyroxine (T4), which is given orally in a single daily dose of 50 to 200 ∝g (19). The initial dose is usually 50 ∝g/day, and this is increased in 50 ∝g/day increments every 3 to 4 weeks. The optimal replacement dose of levothyroxine is determined by monitoring the serum TSH level.
Intravenous thyroxine is recommended for severe hypothyroidism (at least initially), because of the risk of impaired gastrointestinal motility in severe hypothyroidism. One recommended regimen includes an initial intravenous dose of 250 ∝g, followed on the next day by a dose of 100 ∝g, and followed thereafter by a daily dose of 50 ∝g (19).
T3 Replacement Therapy
Because the conversion of T4 to T3 (the active form of thyroid hormone) can be depressed in critically ill patients (18), oral therapy with T3 can be used to supplement thyroxine (T4) replacement therapy. In patients with depressed consciousness, T3 can be given (via NG tube) in a dose of 25 ∝g every 12 hours until the patient awakens (20). Studies evaluating the benefits of T3 supplementation have shown mixed results (15).
A FINAL WORD
Much Ado About Not Much
The major concern with adrenal and thyroid dysfunction in critically ill patients is the possibility of a negative impact on outcomes. However, the following observations suggest that this concern is unfounded.
1. Critical illness-related corticosteroid insufficiency (CIRCI) is common, but the benefits of corticosteroid replacement therapy in critically ill patients are inconsistent (1), and difficult to prove, and this creates doubt about the clinical significance of adrenal suppression in critically ill patients.
2. As for thyroid disorders, hypothyroidism and hyperthyroidism are uncommon in ICU patients, and non-thyroidal effects on thyroid function have no proven influence on clinical outcomes.
Based on these observations, it seems that the adrenal and thyroid dysfunction have very little impact on the overall fate of critically ill patients. (Lots of leaves, but little fruit.)
REFERENCES
Adrenal Insufficiency
1. Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statement from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008; 36L1937–1949.
2. Marik PE. Critical illness-related corticosteroid insufficiency. Chest 2009; 135:181–193.
3. Annane D, Maxime V, Ibrahim F, et al. Diagnosis of adrenal insufficiency in severe sepsis and septic shock. Am J Respir Crit Care Med 2006; 174:1319–1326.
4. Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med 2004; 350:1629–1638.
5. Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med 2009; 360:2328–2339.
Evaluation of Thyroid Function
6. Umpierrez GE. Euthyroid sick syndrome. South Med J 2002; 95:506–513.
7. Peeters RP, Debaveye Y, Fliers E, et al. Changes within the thyroid axis during critical illness. Crit Care Clin 2006; 22:41–55.
8. Dayan CM. Interpretation of thyroid function tests. Lancet 2001; 357:619–624.
9. Karmisholt J, Andersen S, Laurberg P. Variation in thyroid function tests in patients with stable untreated subclinical hypothyroidism. Thyroid 2008; 18:303–308.
10. Hollowell JG, Stachling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988-1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87:489–499.
11. Burman KD, Wartofsky L. Thyroid function in the intensive care unit setting. Crit Care Clin 2001; 17:43–57.
Thyrotoxicosis
12. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: Management guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists. Thyroid 2011; 21:593–646.
13. Klein I. Thyroid hormone and the cardiovascular system. Am J Med 1990; 88:631–637.
14. Migneco A, Ojetti V, Testa A, et al. Management of thyrotoxic crisis. Eur Rev Med Pharmacol Sci 2005; 9:69–74.
Hypothyroidism
15. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults. Endocr Pract 2012; 18:988–1028.
16. Lafayette RA, Costa ME, King AJ. Increased serum creatinine in the absence of renal failure in profound hypothyroidism. Am J Med 1994; 96:298–299.
17. Ladenson PW. Recognition and management of cardiovascular disease related to thyroid dysfunction. Am J Med 1990; 88:638–641.
18. Myers L, Hays J. Myxedema coma. Crit Care Clin 1991; 7:43–56.
19. Toft AD. Thyroxine therapy. New Engl J Med 1994; 331:174–180.
20. McCulloch W, Price P, Hinds CJ, et al. Effects of low dose oral triiodothyronine in myxoedema coma. Intensive Care Med 1985; 11:259–262.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








