FIGURE 46.1 Areas of brain injury and corresponding neurological abnormalities. ‡Indicates deficits involving the same side of the face and the opposite side of the body.
Stroke Mimics
For patients admitted to the hospital with a suspected stroke based on clinical findings, as many as 30% of the patients will have another condition that mimics an acute stroke (11). The most common stroke mimics are seizures, sepsis, metabolic encephalopathies, and space-occupying lesions (in that order) (11). Since stroke is primarily a clinical diagnosis, at least in the first 24–48 hours, stroke mimics are a source of excessive hospital admissions (and thrombolytic therapy) for suspected stroke.
NIH Stroke Scale
The use of a clinical scoring system is recommended to standardize the evaluation of acute stroke (2), and the most validated scoring system is the NIH Stroke Scale (NIHSS). The NIHSS evaluates 11 different aspects of performance, and rates each performance with a number from zero to 3 or 4. The total score is a measure of the severity of illness, and ranges from zero (best performance) to 41 (worst performance). A score of 22 or higher generally indicates a poor prognosis. Trained personnel can complete the NIHSS in less than 5 minutes, and the score can be used to assess the likelihood of an acute stroke (i.e., a stroke is unlikely if the score is 10 or lower) and to follow the clinical course of the illness. (The NIHSS can be downloaded from http://stroke.nih.gov/documents.)
Diagnostic Imaging
The imaging techniques described next have become an integral part of the stroke evaluation, and each technique has a specific role in the evaluation.
Computed Tomography
Noncontrast computed tomography (NCCT) is a reliable method for visualizing intracranial hemorrhage, as shown in Figure 46.2. This reliability is particularly important in the decision to administer thrombolytic therapy, which is contraindicated if NCCT reveals intracranial bleeding. The sensitivity of NCCT for intracranial hemorrhage is close to 100% (5).
NCCT is not a reliable method for visualizing ischemic changes. One-half of ischemic strokes are not apparent on NCCT (12), and the diagnostic yield is even less in the first 24 hours after an acute stroke (when infarct size is the smallest) (13). The nonvalue of CT imaging in the early post-infarct period is demonstrated in Figure 46.3 (13). The CT image on day 3 shows a large area of infarction with mass effect, which is not apparent in the CT image on day 1 (the day of the stroke). These images demonstrate why, in the initial evaluation of suspected stroke, a negative CT scan does not eliminate the possibility of an ischemic stroke.
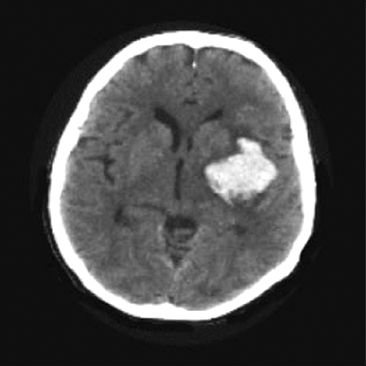
FIGURE 46.2 Noncontrast CT scan showing a high-density mass with adjacent low-density areas in the left hemisphere representing hematoma with adjacent areas of edema. CT is a reliable method for visualizing intracranial hemorrhage.
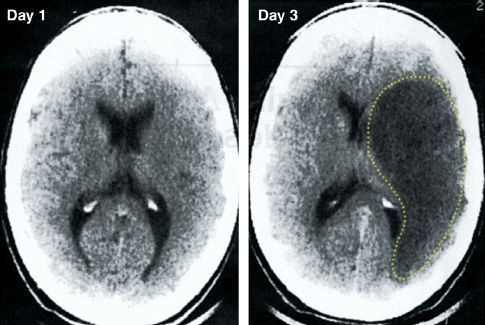
FIGURE 46.3 Noncontrast CT scans from the first and third day following an ischemic stroke. The CT scan on day 1 is unrevealing, while the CT scan on day 3 shows a large hypodense area (outlined by the dotted line) with mass effect, representing extensive tissue destruction with intracerebal edema. Images from Reference 13.
Magnetic Resonance Imaging
MRI with diffusion-weighted imaging (DWI) is the most sensitive and specific technique for the detection of ischemic stroke (2). This technique, which is based on water movement through tissues, can detect ischemic changes within 5–10 minutes after onset (14), and it has a sensitivity of 90% for the detection of ischemic stroke in the early period after stroke onset (5). The appearance of diffusion-weighted MRI in ischemic stroke is shown in Figure 46.4 (15). The image on the left shows a large, hyperdense area representing ischemic changes. (This differs from CT scans, which show ischemic changes as hyopodense areas.) The image on the right is a time-delay technique that detects regions of hypoperfusion using the adjacent color palette. If the ischemic areas on the DWI image are digitally subtracted from the areas of hypoperfusion in the time-delay image, the remaining colored areas on the time-delay map would represent areas of threatened infarction. This digital subtraction technique allows an assessment of continued risk in patients with acute ischemic stroke.
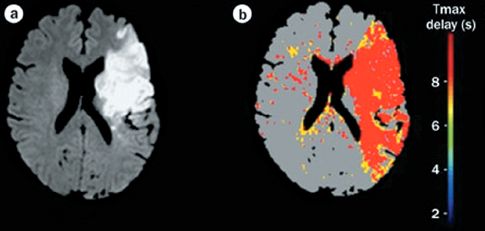
FIGURE 46.4 Diffusion-weighted MRI showing a large area of ischemic change (image on the left). The colored image on the right is a time-delay map, which shows areas of hypoperfusion in red and yellow. Digital subtraction of the ischemic area (image on the left) from the area of hypoperfusion (image on the right) would reveal the areas of threatened infarction. Images from Reference 15.
Echocardiography
The principal role of echocardiography in acute stroke includes the following:
1. Identify a source of cerebral emboli when ischemic stroke is associated with atrial fibrillation, acute MI, or left-sided endocarditis.
2. Identify a patent foramen ovale in patients with ischemic stroke and recent or prior thromboembolism.
THROMBOLYTIC THERAPY
When the initial evaluation identifies a patient with suspected acute stroke, the next step is to determine if the patient is a candidate for thrombolytic therapy.
Selection Criteria
The selection criteria for thrombolytic therapy in ischemic stroke are presented as a checklist in Table 46.1. Some comments about the criteria are presented next.
Time Restriction
The use of thrombolytic therapy in ischemic stroke was prompted by a single study (16), which showed that a 60-minute infusion of recombinant tissue plasminogen activator (tPA) was associated with improved neurologic recovery (not survival), but only when the drug infusion started within 3 hours after the onset of symptoms. The FDA subsequently approved the use of tPA in ischemic stroke (in 1996), but with the restriction that drug administration must be started within 3 hours of symptom onset. This 3-hour time restriction has limited the use of thrombolytic therapy in ischemic stroke; i.e., surveys indicate that only 2% of patients with ischemic stroke receive thrombolytic therapy (17).
Table 46.1 Checklist for Thrombolytic Therapy in Ischemic Stroke

EXPANDED TIME LIMIT: A more recent clinical study has shown that thrombolytic therapy started between 3 and 4.5 hours after the onset of ischemic stroke also improves neurologic recovery (18). Based on these results, the time for initiating thrombolytic therapy has recently been expanded to 4.5 hours after the onset of ischemic stroke (2). There are additional exclusion criteria for the expanded time limit, and these are included in Table 46.1.
Why adhere to these time restrictions? Because about 6% of patients who receive thrombolytic therapy for ischemic stroke will suffer from an intracerebral hemorrhage, so evidence of benefit is necessary to justify this therapy.
Time of Stroke Onset
The time restriction for thrombolytic therapy makes it imperative to pinpoint the time when the stroke began (i.e., became symptomatic). This can be difficult, because patients are unable to provide a reliable history and, in many cases, the onset of symptoms is not witnessed (or occurs during sleep).
Hypertension
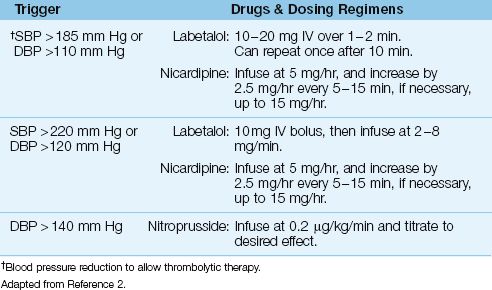
One of the exclusion criteria for thrombolytic therapy is an elevated blood pressure; i.e., a systolic pressure ≥185 mm Hg, or a diastolic pressure ≥110 mm Hg (see Table 46.1). If the patient is otherwise a candidate for thrombolytic therapy, the blood pressure can be lowered to qualify for thrombolytic therapy using the drug regimens shown in Table 46.2 (1). The methods and concerns with blood pressure reduction in acute stroke are described in the last section of the chapter. If the blood pressure reduction is successful and the patient receives thrombolytic therapy, the blood pressure should be maintained at <180/105 for the next few days to limit the risk of intracranial hemorrhage.
Table 46.2 Blood Pressure Control in Acute Stroke
Thrombolytic Regimen
Thrombolytic therapy should be initiated as soon as possible, because earlier initiation is associated with better outcomes (2). Recombinant tissue plasminogen activator (tPA) is the only thrombolytic agent approved for use in acute stroke.
Dosing Regimen: The dose of tPA is 0.9 mg/kg, up to a maximum dose of 90 mg. Ten percent of the dose is given IV over 1–2 minutes, and the remainder is infused over 60 minutes (2).
The infusion should be stopped for any signs of possible intracerebral hemorrhage, such as a deteriorating neurological status, a sudden rise in blood pressure, or a complaint of headache. After the infusion is stopped, an emergent CT scan (without contrast) should be obtained. Following successful completion of the thrombolytic regimen, patients are typically admitted to an ICU for 24 hours. The administration of any anticoagulant or antiplatelet agent is contraindicated for the first 24 hours after thrombolytic therapy.
Antithrombotic Therapy
Heparin
Several studies have failed to show a beneficial effect of heparin anticoagulation in ischemic stroke (2). This lack of benefit, combined with the risks associated with heparin (i.e., bleeding and thrombocytopenia) is the reason that heparin anticoagulation is not recommended in ischemic stroke (2). The only recommended use of heparin in acute stroke is for prevention of thromboembolism (2).
Aspirin
Despite the apparent lack of benefit, aspirin therapy is recommended as a routine measure in ischemic stroke (2). The initial dose is 325 mg (oral), which is given 24–48 hours after stroke onset (or after thrombolytic therapy), and the daily maintenance dose is 75–150 mg (2). Additional antiplatelet agents are not recommended.
PROTECTIVE MEASURES
The measures described in this section are designed to protect the brain from further injury following an acute stroke.
Oxygen Therapy
Oxygen inhalation has been a routine practice in patients with ischemic stroke, even when arterial oxygenation is adequate. This practice has no proven benefit (19), and it neglects the toxic effects of oxygen metabolites (especially the participation of superoxide radicals in reperfusion injury), and the fact that oxygen promotes cerebral vasoconstriction (20).
The latest guidelines on stroke management acknowledge the lack of evidence that oxygen breathing is beneficial in patients with ischemic stroke, and recommend supplemental oxygen only when the arterial O2 saturation falls below 94% (2). This parallels the latest recommendations for oxygen therapy in acute coronary syndromes (see page 306). Although the threshold for oxygen breathing could be lowered to 90%, the new recommendations are a step in the right direction.
Hypertension
Hypertension is reported in 60–65% of patients with acute stroke (21), and is attributed to several factors, including activation of the sympathetic nervous system, cerebral edema, and a prior history of hypertension. Blood pressures usually return to baseline levels in 2–3 days. Patients with stroke-associated hypertension have more extensive neurologic def-icits, but blood pressure reduction is not routinely recommended (2). The indications for blood pressure reduction include a systolic pressure >220 mm Hg, a diastolic blood pressure >120 mm Hg, or a complication of hypertension (e.g., acute MI).
Treatment Regimens
Table 46.2 shows the recommended drugs and dosing regimens for blood pressure reduction in patients with acute stroke. Labetalol (a combined β, α-adrenergic receptor antagonist) and nicardipine (a calcium channel blocker) share the ability to decrease blood pressure while preserving cardiac output (and cerebral blood flow). Labetalol is probably the preferred drug because it does not cause tachycardia, but there are no studies comparing these drugs for blood pressure control in acute stroke. Sodium nitroprusside is recommended for severe hypertension (diastolic BP >140 mm Hg) (2), but nitroprusside infusions are accompanied by an increase in intracranial pressure (22), which is not a desirable condition in the patient with ischemic brain injury.
Fever
Fever develops within 48 hours in 30% of patients with acute stroke (2), and the presence of fever is associated with worse clinical outcomes (23).
Source of Fever
Fever typically appears within 48 hours after stroke onset (24), which suggests a noninfectious origin (e.g., from tissue necrosis or intracerebral blood). However, some studies have found infections in a majority of patients with stroke-related fever (25). Therefore, stroke-related fever should be evaluated as potentially infectious in origin.
Antipyresis
There is convincing evidence from animal studies that fever is harmful for ischemic brain tissue (27), and thus antipyretic therapy is justified for stroke-related fever. Antipyretic therapy is described in Chapter 43.
A FINAL WORD
Where’s the Beef?
The success of thrombolytic therapy in coronary occlusive syndromes created high expectations for thrombolytic therapy in acute, ischemic stroke, and these expectations prompted a massive effort to create “stroke centers” in major hospitals, each with a “stroke team” to direct the management of acute stroke. The following is an accounting of what this effort has accomplished.
| Number of strokes each year in the United States | 700,000 |
| Number of ischemic strokes (88% ) | 616,000 |
| Number of stroke patients who receive lytic therapy (2%) | 12,320 |
| Number of patients who benefit from lytic therapy (1 in 9) | 1,369 |
| Percent of strokes that benefit from lytic therapy (1369/700,000) | 0.2% |
Enough said.
REFERENCES
1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics – 2013 update: A report from the American Heart Association. Circulation 2013; 127:e6–e245.
Clinical Practice Guideline
2. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke. A guideline for healthcare professionals from The American Heart Association/American Stroke Association. Stroke 2013; 44:1–78.
Definitions
3. Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke 1990; 21:637–676.
4. Kizer JR, Devereux RB. Clinical practice. Patent foramen ovale in young adults with unexplained stroke. N Engl J Med 2005; 353:2361–2372.
5. Culebras A, Kase CS, Masdeu JC, et al. Practice guidelines for the use of imaging in transient ischemic attacks and acute stroke. A report of the Stroke Council, American Heart Association. Stroke 1997; 28:1480–1497.
6. Ovbiagele B, Kidwell CS, Saver JL. Epidemiological impact in the United States of a tissue-based definition of transient ischemic attack. Stroke 2003; 34:919–924.
Initial Evaluation
7. Saver JL. Time is brain—quantified. Stroke 2006; 37:263–266.
8. Bamford J. Clinical examination in diagnosis and subclassification of stroke. Lancet 1992; 339:400–402.
9. Atchison JW, Pellegrino M, Herbers P, et al. Hepatic encephalopathy mimicking stroke. A case report. Am J Phys Med Rehabil 1992; 71:114–118.
10. Maher J, Young GB. Septic encephalopathy. Intensive Care Med 1993; 8:177–187.
11. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: the brain attack study. Stroke 2006; 37:769–775.
12. Warlow C, Sudlow C, Dennis M, et al. Stroke. Lancet 2003; 362:1211–1224.
13. Graves VB, Partington VB. Imaging evaluation of acute neurologic disease. In: Goodman LR Putman CE, eds. Critical care imaging. 3rd ed. Philadelphia: W.B. Saunders, Co., 1993; 391–409.
14. Moseley ME, Cohen Y, Mintorovich J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med 1990; 14:330–346.
15. Asdaghi N, Coutts SB. Neuroimaging in acute stroke – where does MRI fit in? Nature Rev Neurol 2011; 7:6–7.
Thrombolytic Therapy
16. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333:1581–1587.
17. Caplan LR. Thrombolysis 2004: the good, the bad, and the ugly. Rev Neurol Dis 2004; 1:16–26.
18. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329.
Protective Measures
19. Ronning OM, Guldvog B. Should stroke victims routinely receive supplemental oxygen. A quasi-randomized controlled trial. Stroke 1999; 30:2033–2037.
20. Kety SS, Schmidt CF. The effects of altered tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 1984; 27:484–492.
21. Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med 2007; 25:32–38.
22. Candia GJ, Heros RC, Lavyne MH, et al. Effect of intravenous sodium nitroprusside on cerebral blood flow and intracranial pressure. Neurosurgery 1978; 3:50–53.
23. Reith J, Jorgensen HS, Pedersen PM, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet 1996; 347:422–425.
24. Wrotek SE, Kozak WE, Hess DC, Fagan SC. Treatment of fever after stroke: conflicting evidence. Pharmacotherapy 2011; 31:1085–1091.
25. Grau AJ, Buggle F, Schnitzler P, et al. Fever and infection early after ischemic stroke. J Neurol Sci 1999; 171:115–120.
26. Baena RC, Busto R, Dietrich WD, et al. Hyperthermia delayed by 24 hours aggravates neuronal damage in rat hippocampus following global ischemia. Neurology 1997; 48:768–773.
27. Sulter G, Elting JW, Mauritis N, et al. Acetylsalicylic acid and acetaminophen to combat elevated temperature in acute ischemic stroke. Cerebrovasc Dis 2004; 17:118–122.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








