Man Adapting
1966
Urethral catheters are commonplace in critically ill patients, and surveys indicate that catheter-associated urinary tract infections account for 40% of all hospital-acquired infections in the United States (1). However, these surveys are misleading, because a majority of the catheter-associated infections represent asymptomatic bacteriuria (infection without disease, according to Dubois), and do not require antimicrobial therapy. This chapter describes the current recommendations for the diagnosis and treatment of catheter-associated symptomatic urinary tract infection.
PATHOGENESIS
The presence of a urethral catheter is associated with a 3–8% incidence of bacteriuria (≥105 colony forming units/mL) per day (1). This is assumed to be the result of bacterial migration along the outer surface of the catheter and into the bladder. Bacteria also form biofilms on the inner and outer surface of urethral catheters (2), and these biofilms can serve as a source of continued microbial colonization in the bladder. However, this is not the full story, because direct injection of pathogens into the bladder of healthy subjects does not result in a urinary tract infection (3). Furthermore, the continuous flow of urine that is allowed by bladder drainage catheters should wash away microbes that migrate up the urethra.
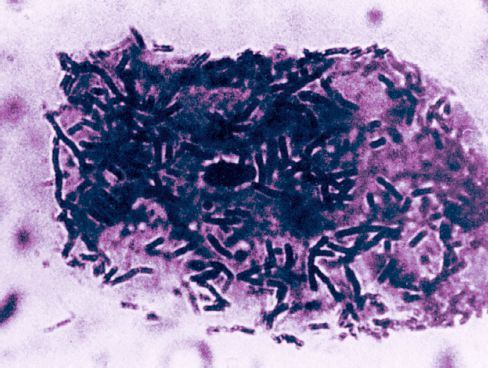
FIGURE 41.1 Photomicrograph showing non-pathogenic Lactobacillus organisms adhering to a bladder epithelial cell. From Reference 3. Image has been digitally enhanced.
Bacterial Adherence
The missing piece of the puzzle is the ability of pathogenic organisms to adhere to the bladder epithelium. The epithelial cells of the bladder are normally coated with non-pathogenic organisms, as shown in Figure 41.1 (4); this prevents the attachment of pathogenic organisms, which is the precipitating event that leads to infections of the lower urinary tract (5). This is the same phenomenon that occurs in colonization of the oral mucosa with pathogenic Gram-negative aerobic bacilli (see Figure 5.5), which serves as a prelude to nosocomial pneumonia. The link between bladder catheters and the change in bacterial adherence is unclear. However, it is possible that an increase in severity of illness is responsible for both the change in bacterial adherence and the need for a bladder catheter. (For more on the role of bacterial adherence in nosocomial infections, see “A Final Word” at the end of the chapter.)
Microbiology
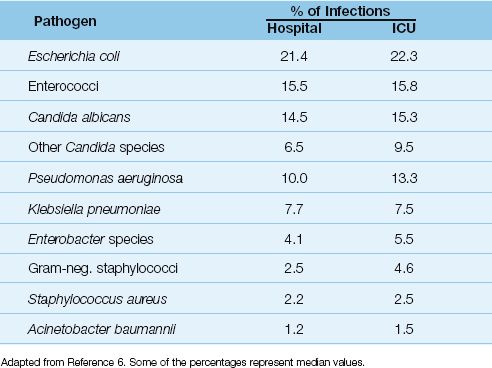
The pathogens isolated in catheter-associated bacteriuria are shown in Table 41.1 (6). The predominant organisms are Gram-negative aerobic bacilli (especially Escherichia coli), enterococci, and Candida species, while staphylococci are infrequent isolates. A single organism predominates in bacteriuria associated with short-term catheterization (<30 days), whereas bacteriuria is often polymicrobial in long-term catheterization (≥30 days).
Table 41.1 Pathogens Isolated in Catheter-Associated Bacteriuria
Prevention
The risk of catheter-associated infection is determined primarily by the duration of catheterization (1), so removing catheters when they are no longer necessary is the single most effective prophylactic measure for catheter-associated infections. Other observations about prevention are summarized below.
1. Cleansing of catheter insertion sites (with antiseptic solutions, antibiotic creams, or soap and water) is NOT recommended because this practice can increase the risk of bacteriuria (1).
2. Prophylaxis with systemic antibiotics is not recommended for preventing infections of the urinary tract (1).
3. Antimicrobial-impregnated urinary catheters (i.e., with silver alloy or nitrofurazone) can reduce the incidence of asymptomatic bacteriuria in short-term catheterization (<1 week) (7), but the benefit in preventing symptomatic urinary tract infections is not clear (1).
DIAGNOSIS AND TREATMENT
The recommendations in this section are taken from the most recent guidelines developed by the Infectious Disease Society of America (1).
Diagnosis
1. In patients with short-term catheters (<30 days), urine specimens for culture can be obtained by sampling through the catheter port or catheter tubing. For patients with long-term catheters (≥30 days), the catheter should be replaced before collecting the urine specimen.
2. Significant bacteriuria in catheterized patients is defined as a urine culture that grows ≥105 colony forming units (cfu) per mL. How-ever, over 90% of patients with significant bacteriuria have no other evidence of infection (asymptomatic bacteriuria) (8).
3. Catheter-associated urinary tract infection (CA-UTI) is defined as a urine culture that grows >103 cfu/mL in a patient with clinical signs of a symptomatic UTI. These can include:
a. Bacteremia with the same organism isolated in blood and urine.
b. New costovertebral tenderness.
c. Rigors.
d. New onset of delirium or depressed consciousness.
e. Increased spasticity in patients with spinal cord injuries.
Common symptoms of UTI such as dysuria and frequency are not relevant in catheterized patients, and the usual signs of infection (fever, leukocytosis) can lack specificity in catheterized patients (see next).
4. The following findings are NOT reliable for the diagnosis of CA-UTI:
a. The presence of fever or leukocytosis.
b. Cloudy urine.
c. The presence of white blood cells in urine (pyuria).
The problem with fever and leukocytosis is that catheterized patients often have another infection that could explain these findings. Furthermore, in one study comparing patients with suspected CA-UTI to patients without CA-UTI where there was no other apparent infection, the incidence of fever and leukocytosis was the same in the two groups of patients (8). The presence of white blood cells in urine (pyuria) does not differentiate between asymptomatic bacteriuria and CA-UTI, but the absence of pyuria can be used as evidence against the diagnosis of CA-UTI (1).
Treatment
1. Screening for, or antibiotic treatment of, asymptomatic bacteriuria is NOT advised unless the patient is scheduled for a urologic procedure that is associated with mucosal bleeding (e.g., transurethral resection of the prostate) (9). These recommendations are based on the following observations: (a) few cases of asymptomatic bacteriuria progress to CA-UTI, (b) antibiotic therapy does not reduce the incidence of CA-UTI, and (3) antibiotic therapy promotes the emergence of resistant organisms.
2. Empiric antibiotics are recommended for patients with suspected CA-UTI. Single agent therapy with piperacillin-tazobactam or a carbapenem (imipenem or meropenem) is recommended, while levofloxacin (750 mg IV once daily) is a second-line agent (10).
3. If the diagnosis of CA-UTI is confirmed by urine culture, antibiotic therapy should be adjusted according to the organism isolated and the reported sensitivities. Catheters that have been in place for >2 weeks should be replaced.
4. The duration of antibiotic therapy for CA-UTI should be 7 days for patients who respond promptly, and 10–14 days for patients with a delayed response.
CANDIDURIA
The presence of Candida species in urine usually represents colonization in patients with indwelling urethral catheters, but candiduria can also be a sign of disseminated candidiasis (i.e., the candiduria being the result, not the cause, of the disseminated candidiasis). Disseminated candidiasis can be an elusive diagnosis because blood cultures are unrevealing in more than 50% of cases (11), and candiduria may be the only evidence of disseminated disease. The clinical condition of the patient becomes an important factor in the approach to candiduria in the ICU.
Microbiology
In cases of candiduria, the colony count has no predictive value for identifying renal or disseminated candidiasis (11). The most frequent isolate is Candida albicans (about 50% of cases), followed by Candida glabrata (about 15% of cases) (11). The latter organism is notable for resistance to the antifungal agent fluconazole.
Asymptomatic Candiduria
Asymptomatic candiduria does not require treatment unless the patient is neutropenic (12,13). Removal of the catheter is always advised, when possible, because this can eradicate candiduria in 40% of cases (13). Repeat urine cultures are recommended, and persistent candiduria in high-risk (immunosuppressed) patients should be investigated with blood cultures and imaging studies of the kidneys.
In neutropenic patients with asymptomatic candiduria, the recommended prophylaxis includes caspofungin: 70 mg IV as a loading dose, followed by 50 mg IV daily (12).
Symptomatic Candiduria
Candiduria that is associated with fever, suprapubic tenderness, or costovertebral tenderness requires antifungal therapy as well as blood cultures and imaging studies of the kidneys (with ultrasound or computed tomography) to search for renal abscesses or evidence of urinary tract obstruction. Renal candidiasis is usually a consequence of disseminated candidiasis (11).
The treatment for symptomatic candiduria is summarized below.
1. The recommended treatment for Candida cystitis and pyelonephritis is fluconazole (PO or IV): 400 mg daily for 2 weeks (14). This regimen can eradicate infections caused by organisms that are resistant to fluconazole (i.e., C. glabrata and C. krusei) because fluconazole is concentrated in the urine. Decreasing the dose of fluconazole in renal insufficiency (which is normally recommended) is not advised for Candida UTIs because this would decrease urinary concentrations of fluconazole to subtherapeutic levels (14).
2. Candida UTIs that do not respond to fluconazole can be treated with oral flucytosine: 25 mg/kg every 6 hours (with adjustments for renal insufficiency) for 7–10 days (14). The duration of treatment is limited with this drug because it causes bone marrow suppression and mucosal injury in the GI tract.
3. For candiduria that is associated with hemodynamic instability or progressive multiorgan dysfunction (i.e., when disseminated candidiasis is suspected) the recommended treatment is IV fluconazole in a loading dose of 800 mg followed by 400 mg daily (14).
A FINAL WORD
Bacterial Adherence
The unifying feature in nosocomial infections that involve the gastrointestinal, respiratory, and urinary tracts is a change in the character of microbes that adhere to epithelial surfaces. In healthy subjects, the epithelial surfaces in the mouth, GI tract, and urinary tract are covered by harmless, commensal organisms, but in patients who develop severe or chronic illness, these surfaces are covered with pathogenic organisms, and this serves as a prelude to nosocomial infections. Of interest in this regard is a study conducted in patients with spinal cord injuries and long-term urinary catheters, where injection of non-pathogenic E. coli into the bladder was associated with 50% fewer urinary tract infections (15).
However, the population of epithelial surfaces is not just a matter of “territorial imperative” (where one population takes over, or defends, a territory) but is the result of receptors on epithelial cells that bind to specific groups of microorganisms. A change in the configuration of these receptors allows pathogens to bind to epithelial surfaces, and this is the precipitating event that leads to nosocomial infections. As such, we need to study how microbes bind to epithelial surfaces if we want to eliminate the threat of nosocomial infections.
REFERENCES
1. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infections in adults: 2009 international clinical practice guidelines from the Infectious Disease Society of America. Clin Infect Dis 2010; 50:625–663.
2. Ganderton L, Chawla J, Winters C, et al. Scanning electron microscopy of bacterial biofilms on indwelling bladder catheters. Eur J Clin Microbiol Infect Dis 1992; 11:789–796.
3. Howard RJ. Host defense against infection – Part 1. Curr Probl Surg 1980; 27:267–316.
4. Sobel JD. Pathogenesis of urinary tract infections: host defenses. Infect Dis Clin North Am 1987; 1:751–772.
5. Daifuku R, Stamm WE. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N Engl J Med 1986; 314:1208–1213.
6. Shuman EK, Chenoweth CE. Recognition and prevention of healthcare-associated urinary tract infections in the intensive care unit. Crit Care Med 2010; 38(Suppl):S373–S379.
7. Schumm K, Lam TB. Types of urethral catheters for management of short-term voiding problems in hospitalized adults. Cochrane Database Syst Rev 2008:CD004013.
8. Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic. Arch Intern Med 2000; 160:678–682.
9. Nicolle LE, Bradley S, Colgan R, et al. Infectious Disease Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
10. Gilbert DN, Moellering RC, Eliopoulis, et al, eds. The Sanford guide to antimicrobial therapy, 2009. 39th ed. Sperryville, VA: Antimicrobial Therapy, Inc, 2009:31.
11. Hollenbach E. To treat or not to treat – critically ill patients with candiduria. Mycoses 2008; 51(Suppl 2):12–24.
12. Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Disease Society of America. Clin Infect Dis 2009; 48:503–525.
13. Sobel JD, Kauffman CA, McKinsey D, et al. Candiduria: a randomized double-blind study of treatment with fluconazole or placebo. Clin Infect Dis 2000; 30:19–24.
14. Fisher JF, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infections – treatment. Clin Infect Dis 2011; 52(Suppl 6):S457–S466.
15. Darouiche RO, Thornby JI, Cerra-Stewart C, et al. Bacterial interference for prevention of urinary tract infection: a prospective, randomized, placebo-controlled, double-blind pilot trial. Clin Infect Dis 2005; 41:1531–1534.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








