Nonoperating room (NOR) locations are remote from a hospital’s main operating room sites.
 A significant number of the procedures performed in NOR locations require anesthesia or sedation.
A significant number of the procedures performed in NOR locations require anesthesia or sedation.
 A three-step approach that considers the patient, the procedure, and the environment is useful in considering any nonoperating room anesthetic.
A three-step approach that considers the patient, the procedure, and the environment is useful in considering any nonoperating room anesthetic.
 Patient considerations include whether the patient will tolerate sedation or require general anesthesia, the ASA classification, significant comorbidities, and the level of monitoring.
Patient considerations include whether the patient will tolerate sedation or require general anesthesia, the ASA classification, significant comorbidities, and the level of monitoring.
 Procedural considerations are both general (e.g., duration, position, and level of discomfort) and specific to individual specialties.
Procedural considerations are both general (e.g., duration, position, and level of discomfort) and specific to individual specialties.
 The American Society of Anesthesiologists (ASA) has defined guidelines to be applied to the administration of anesthesia at nonoperating room locations.
The American Society of Anesthesiologists (ASA) has defined guidelines to be applied to the administration of anesthesia at nonoperating room locations.
 Patients should receive the same standard of care at a NOR site as they do in the operating room.
Patients should receive the same standard of care at a NOR site as they do in the operating room.
 The anesthetic and monitoring equipment used for NORA must meet the same standards as equipment provided in the operating room.
The anesthetic and monitoring equipment used for NORA must meet the same standards as equipment provided in the operating room.
 Following NORA, the patient should be transported to an appropriate postanesthesia care unit, accompanied and monitored by anesthesia-trained personnel.
Following NORA, the patient should be transported to an appropriate postanesthesia care unit, accompanied and monitored by anesthesia-trained personnel.
 Environmental considerations include hazards such as radiation and the side effects of intravenous contrast agents.
Environmental considerations include hazards such as radiation and the side effects of intravenous contrast agents.
Multimedia
 Anesthesia in Remote Locations
Anesthesia in Remote Locations
GENERAL PRINCIPLES
Nonoperating Room Anesthesia (NORA) refers to anesthesia that is provided at locations remote from the familiar territory of the traditional operating room. These locations include radiology departments, endoscopy suites, magnetic resonance imaging (MRI) scanners, or dental clinics. This chapter will consider these locations. Discussion of anesthesia for surgical procedures performed in stand-alone ambulatory centers, or offices, is addressed in Chapters 30 and 31. Anesthesia and analgesia provided for labor and delivery is in Chapter 40
Room Anesthesia (NORA) refers to anesthesia that is provided at locations remote from the familiar territory of the traditional operating room. These locations include radiology departments, endoscopy suites, magnetic resonance imaging (MRI) scanners, or dental clinics. This chapter will consider these locations. Discussion of anesthesia for surgical procedures performed in stand-alone ambulatory centers, or offices, is addressed in Chapters 30 and 31. Anesthesia and analgesia provided for labor and delivery is in Chapter 40 . Nonoperating room (NOR) cases account for a significant proportion of the procedural work of hospitals. Increasingly, the patients and/or the proceduralists require or request anesthesia or sedation to facilitate these procedures.
. Nonoperating room (NOR) cases account for a significant proportion of the procedural work of hospitals. Increasingly, the patients and/or the proceduralists require or request anesthesia or sedation to facilitate these procedures.
THE THREE-STEP APPROACH TO NORA
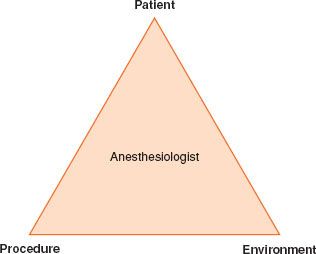
Away  from the operating room, the anesthesiologist may lack familiar equipment and staff experienced in the care of the anesthetized patient. NORA; therefore, presents unique challenges and a systematic approach using the simple three-step paradigm “the PATIENT, the PROCEDURE, and the ENVIRONMENT” is recommended (Fig. 32-1).
from the operating room, the anesthesiologist may lack familiar equipment and staff experienced in the care of the anesthetized patient. NORA; therefore, presents unique challenges and a systematic approach using the simple three-step paradigm “the PATIENT, the PROCEDURE, and the ENVIRONMENT” is recommended (Fig. 32-1).
FIGURE 32-1. A three-step paradigm for NORA.
The Patient
Patients may require sedation or anesthesia to tolerate NOR procedures for a number of reasons (Table 32-1). Children are more likely to require sedation or anesthesia for diagnostic and therapeutic procedures. Patients who are too ill to tolerate a major surgical procedure, but who may be able to undergo a palliative, less-invasive procedure also represent a challenge for the NOR anesthesiologist.  All patients presenting for NORA require a thorough preanesthetic assessment and the development of a sound anesthetic plan with appropriate levels of monitoring.
All patients presenting for NORA require a thorough preanesthetic assessment and the development of a sound anesthetic plan with appropriate levels of monitoring.
TABLE 32-1. PATIENT FACTORS REQUIRING SEDATION OR ANESTHESIA FOR NONOPERATING ROOM PROCEDURES

The Procedure
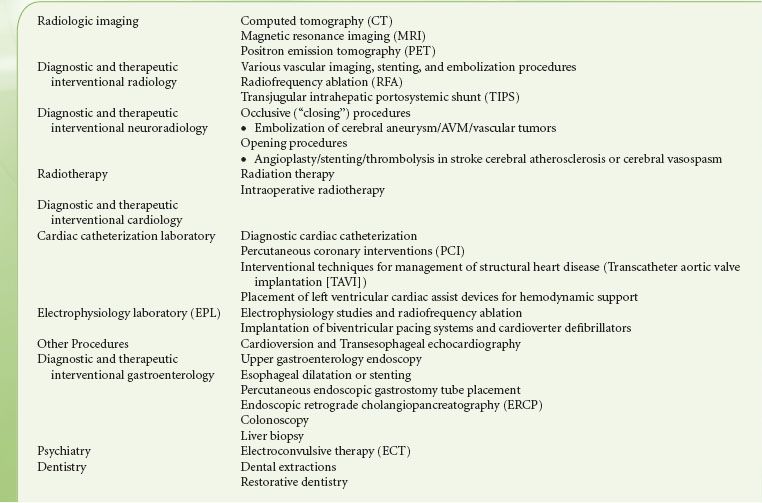
Common NOR procedures for which the patient may require anesthesia or sedation are listed (Table 32-2) . The anesthesiologist must understand the nature of the procedure, including the position of the patient, how painful the procedure will be, and how long it will last. The optimum anesthesia plan provides safe patient care and facilitates the procedure. Discussions with the proceduralist must include contingencies for emergencies and adverse outcomes.
. The anesthesiologist must understand the nature of the procedure, including the position of the patient, how painful the procedure will be, and how long it will last. The optimum anesthesia plan provides safe patient care and facilitates the procedure. Discussions with the proceduralist must include contingencies for emergencies and adverse outcomes.
TABLE 32-2. COMMON NONOPERATING ROOM ANESTHESIA PROCEDURES
The Environment
The American Society of Anesthesiologists  (ASA) has developed standards for NORA.1 Prior to the anesthetic, the presence and proper functioning of all equipment needed for safe patient care must be established; this is described in Table 32-3. The location of immediately available resuscitation equipment should be noted and protocols developed with the local staff for dealing with emergencies, including cardiopulmonary resuscitation and the management of anaphylaxis.
(ASA) has developed standards for NORA.1 Prior to the anesthetic, the presence and proper functioning of all equipment needed for safe patient care must be established; this is described in Table 32-3. The location of immediately available resuscitation equipment should be noted and protocols developed with the local staff for dealing with emergencies, including cardiopulmonary resuscitation and the management of anaphylaxis.
Anesthesia Equipment and Monitors
In some NOR locations, anesthesia machines and monitors are provided; in others, it may be necessary to bring anesthesia equipment to the location. Small, portable anesthesia machines and monitors are available and recommended in these situations. Anesthesia machines and monitors that remain in a NOR location need to undergo routine maintenance. Infrequent use may result in degradation of equipment and the use of preprocedural checks, preferably with a standardized checklist, cannot be overemphasized before embarking on NORA. If more advanced monitors (e.g., an arterial line, central venous pressure, or intracranial pressure [ICP] monitoring) are required, these devices should be readily available. A preprepared cart containing essential equipment that is checked and restocked after each case is recommended.
PATIENT SAFETY IN NONOPERATING ROOM ANESTHESIA
Patient safety is of utmost importance in all types of anesthesia, not least NORA.
Adverse Events
Significant adverse events occur infrequently in NOR locations, although the large multicenter studies needed to determine their true incidence are lacking.2 The comparative safety of sedation administered by anesthesiology versus nonanesthesiology trained providers is not known.2 Adverse events occurring during NORA have been investigated using the ASA closed claims database.3 In this analysis, NORA was associated with a higher number of deaths compared to anesthesia conducted in the operating room and 50% of these were associated with monitored anesthesia care (MAC). Respiratory depression secondary to over-sedation was the most common type of adverse event in the closed claims study.3 This is consistent with the findings from other studies where the majority of adverse events related to sedation in NORA are due to the airway and respiratory insufficiencies.2 Capnography provides an earlier monitor of impending respiratory depression during sedation and is suggested as an important addition to pulse oximetry.4,5,6
Preprocedural Checklists
The use of checklists and pre- and postprocedural team briefings has been broadly embraced in operating room practice and emerging evidence points toward improved patient outcomes when checklists are employed.7 Similar systems should be adopted in NOR sites and recently a checklist has been proposed for use in interventional radiology suites.8
Standards of Care for Nonoperating Room Anesthesia
The  ASA has published a number of guidelines and standards of care for preanesthesia9 and postanesthesia care,10 basic monitoring standards,4 and MAC.
ASA has published a number of guidelines and standards of care for preanesthesia9 and postanesthesia care,10 basic monitoring standards,4 and MAC. 11 These standards apply to patients being cared for in all NOR sites in the same way as they do in the operating rooms.
11 These standards apply to patients being cared for in all NOR sites in the same way as they do in the operating rooms.
At the conclusion of the NOR procedure, the patient should be transported to a recovery area by a member of the anesthesia team who must provide a full verbal report to the recovery nursing staff.10 The recovery area should be equipped to the same standards as for postoperative patients .
.
Patient Transfer
Sick, unstable patients may be transferred back and forth between the intensive care unit, the operating rooms and NOR locations for imaging or diagnostic procedures. During transport the patient should be accompanied by a member of the anesthesia team to evaluate, monitor, and support the patient’s medical condition. These patients are often mechanically ventilated and receiving a number of drug infusions for both sedation and hemodynamic support. Portable ventilators are useful for transport; these are often oxygen powered, and adequate supplies of oxygen must be available for the transfer. A manual self-inflating bag is essential in the event of ventilator failure. Infusion pumps and portable monitors should have adequate battery power for transit. The anesthesiologist should carry spare anesthetic and emergency drugs, equipment for intubation or reintubation, portable suction, and if the patient’s condition requires, a portable defibrillator. It is vital to notify the destination area that the patient is in transit; so appropriate preparations to receive the patient can be made in advance. It is also useful to send personnel ahead to secure the elevators to prevent delays during transfer.
SEDATION AND ANESTHESIA
Definition of Sedation and Anesthesia
Many NOR procedures are performed under “sedation” or “MAC”. A consistent definition of these terms is essential for clear communications between the various stakeholders involved in provision of NORA. On January 14, 2011, the Centers for Medicare and Medicaid Services (CMS) issued a revision to Interpretive Guideline (IG) for Hospitals No. 482.52 concerning anesthesia services.12 This revised guideline places the responsibility and oversight for all anesthesia services under the direction of one suitably qualified individual, the “director of anesthesia services.” IG 482.52 defines anesthesia as “the administration of a medication to produce a blunting or loss of, pain perception (analgesia); voluntary and involuntary movements; autonomic function; and memory and/or consciousness.”
The Continuum of Anesthesia
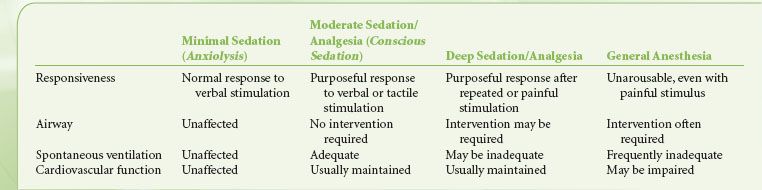
Anesthesia exists along a continuum and the transition from minimal sedation to general anesthesia is not clear-cut6,11 (Table 32-4). As sedation deepens, it is important to recognize the progressive blunting and loss of airway reflexes and patency, together with depression of spontaneous ventilation and cardiovascular function. The individual responsiveness of patients to different sedative agents varies, as do the levels of stimulation during the course of a procedure. Consequently, during the course of a NOR procedure under sedation, the patient may drift to a deeper level than the one intended, including transitioning into general anesthesia with loss of the airway. In any circumstances where a particular level of sedation is being provided, services must be immediately available to rescue a patient from a deeper than intended level of sedation or general anesthesia.6,11,12
ENVIRONMENTAL CONSIDERATIONS FOR NONOPERATING ROOM ANESTHESIA
X-rays and Fluoroscopy
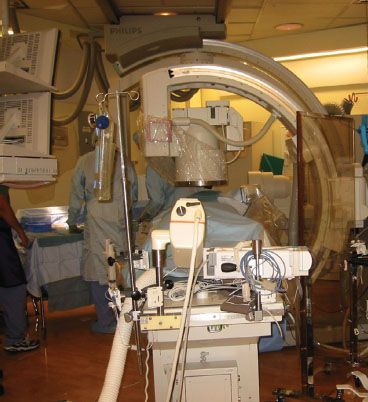
When electrons are accelerated by means of a high voltage to a high velocity in a vacuum tube, a current is created that collides with a metal target producing X-rays. In medical X-ray tubes, the target is usually tungsten or a more crack-resistant alloy of rhenium (5%) and tungsten (95%). X-ray production is determined by, and directly proportional to, the tube current and the voltage. Fluoroscopy is a technique used to obtain real-time moving images of the internal structures. The patient is positioned between the X-ray source and a fluorescent screen; by coupling the fluoroscope to an X-ray image intensifier and a video camera, the images can be recorded and played on a monitor. Fluoroscopy is widely used in many NOR locations including interventional radiology, cardiac catheterization, and electrophysiologic procedures and in the gastroenterology suite. Large, C-shaped, mobile fluoroscopy devices (C-arms) are used to provide images in multiple dimensions. The C-arm moves back and forth around the patient during the procedure, taking up large amounts of space, limiting access to the patient and serving as a means of dislodging intravenous lines and endotracheal tubes (Fig. 32-2).
FIGURE 32-2. A radiology suite showing a C-arm and the high density of equipment that may separate the anesthesiologist from the patient.
Hazards of Ionizing Radiation
The use of fluoroscopy for both diagnostic and interventional procedures continues to increase and with it the risks exposure of patients and staff to the effects of ionizing radiation. Radiation exposure for patients varies depending on the type of procedure as well as patient and operator-related factors.13,14 Occupational exposure for staff including anesthesiologists working in radiology suites is an important consideration. The Cardiovascular and Interventional Society of Europe (CIRSE) Standards of Practice Committee and the Society of Interventional Radiology (SIR) Safety and Health Committee have recently published guidelines on occupational protection from radiation.15 A number of terms are used to define exposure to radiation; these are summarized in Table 32-5. Exposure from fluoroscopy is between 100 and 1,000 greater than from simple X-rays. For example, exposure from a simple chest X-ray is 0.02 mSv while pulmonary angiography produces 20 to 40 mSv.16
The effects of ionizing radiation on biologic tissues are classified as deterministic (dose related causing cell death and tissue damage) and stochastic (development of cancer from direct DNA ionization or the creation of hydroxyl radicals from X-ray interactions with water molecules). Protective measures to reduce patient exposure to radiation should always be taken. Staff, including the anesthesiologists must be aware of the hazards of occupational exposure to ionizing radiation.15 Exposure to ionizing radiation may come from direct exposure and scatter. Patients are subjected to direct exposure where the beam enters the skin. Staff working in fluoroscopy suites are more at risk from scattered radiation, and as a general rule the exposure to staff is 1/1,000th the entrance skin exposure at 1 m from the fluoroscopy tube. Staff exposure to radiation can be minimized by14:
must be aware of the hazards of occupational exposure to ionizing radiation.15 Exposure to ionizing radiation may come from direct exposure and scatter. Patients are subjected to direct exposure where the beam enters the skin. Staff working in fluoroscopy suites are more at risk from scattered radiation, and as a general rule the exposure to staff is 1/1,000th the entrance skin exposure at 1 m from the fluoroscopy tube. Staff exposure to radiation can be minimized by14:
1. Limiting the time of exposure to radiation.
2. Increasing the distance from the source of radiation. (Dose rates increase or decrease according to the inverse square of the distance from the source.)
3. Using protective shielding (lead-lined garments or fixed and movable shields).
Lead aprons, thyroid shields, and leaded eyeglasses are bulky and significantly increase staff fatigue. Recent studies have demonstrated that anesthesiologists are at equal risk of developing cataracts as neuroradiologists, and that the radiation may even be directed away from the neuroradiologists and toward the anesthesiologist.17 Anesthesiologists must use appropriate leaded eye protection to minimize this risk. Anesthesiology staff should use movable or fixed lead-lined glass shields so that they can gain easy access to their patients while at the same time protect themselves from radiation.
4. Using dosimeters.
National organizations set standards that limit the exposure of personnel to radiation doses, all personnel working in sites where there may be exposure to radiation should wear dosimeters to monitor their exposure. The International Commission on Radiological Protection (ICRP) recommends that staff must wear two dosimeters, one under the apron and one at collar level above the lead apron.18 In the United States, the National Council on Radiation Protection and Measurements (NCRP) recommends an occupational limit of 50 mSv in any 1 year and a lifetime limit of 10 mSv multiplied by the individual’s age in years.19 Anesthesiologists who are involved in NOR cases that involve exposure to ionizing radiation must wear dosimeters and be included in the institution’s radiation safety section or medical physics service regular monitoring to ensure that dose limits are not exceeded.15
Intravenous Contrast Agents
Intravenous contrast agents are commonly used in radiologic and MRI to enhance vascular imaging.20 Radiologic contrast media are iodinated compounds classified according to their osmolarity (high, low, or iso-osmolar) and their ionicity (ionic or nonionic). Nonionic contrast agents cause less discomfort on injection and have a lower incidence of adverse reactions. MRI contrast agents are also divided into ionic and nonionic compounds. They are chelated metal complexes containing gadolinium, iron, or manganese.
Adverse reactions to contrast agents may be divided into renal adverse reactions and hypersensitivity reactions.
Renal Adverse Reactions
Contrast agents are eliminated via the kidneys, and contrast-induced nephropathy (CIN) occurs with an incidence of 7% to 15%21; intra-arterial injection is associated with a higher incidence of CIN than intravenous route.20 CIN is defined as an increase in serum creatinine of 0.5 mg/dL or a 25% increase from the baseline within the first 24 hours, peaking at 5 days. It is the third leading cause of hospital-acquired acute renal failure.22 Risk factors for CIN include history of renal disease, prior renal surgery, proteinuria, diabetes mellitus, hypertension, gout, and use of nephrotoxic drugs.20 In the setting of CIN, metformin can cause lactic acidosis and should be discontinued prior to the patient receiving intravenous contrast agents. Iso-osmolar agents have the lowest risk of CIN and iodixanol has the lowest incidence of CIN in patients with renal impairment.23 Preventative measures to avoid CIN include adequate hydration, maintaining a good urine output, and using sodium bicarbonate infusions to improve elimination of the contrast agent. The efficacy of N-acetylcysteine and other agents such as fenoldopam, dopamine, calcium-channel blockers, atrial natriuretic peptide, and L-arginine in mitigating CIN has not been proven.21
Hypersensitivity Reactions
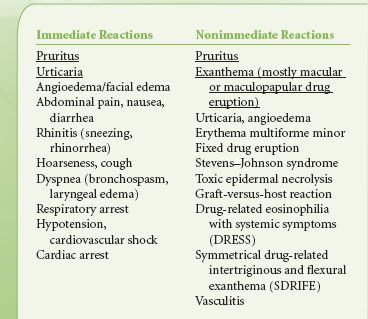
Hypersensitivity reactions to contrast media are divided into immediate (<1 hour) and nonimmediate (>1 hour) reactions.24 Mild immediate reactions occur in about 0.5% to 3% and severe reactions occur in 0.01% to 0.04%. Fatal hypersensitivity reactions may occur in about 1 per 100,000 contrast administrations.24 The frequency of nonimmediate reactions is much more variable (0.5% to 23%) related partly to difficulty in determining whether symptoms relate to contrast agents or not.24 The clinical manifestations of various hypersensitivity reactions to contrast media are outlined in Table 32-6.25 Although widely used, the effectiveness of corticosteroids and antihistamines in preventing hypersensitivity reactions to contrast agents in unselected patients is doubtful.26 Treatment of severe hypersensitivity reactions includes discontinuing the causative agent and supportive therapy; oxygen, airway securement, cardiovascular support with fluids, vasopressors, and inotropes, and if required, bronchodilators. Reactions to gadolinium-based contrast agents used for MRI are less frequent than to iodinated contrast agents with hypersensitivity to gadolinium-containing agents occurring in 5.9 per 10,000 injections. The rate is higher (13 per 10,000) in patients undergoing abdominal MRI examinations. Severe reactions occur 1:10,000 to 1:40,000 and the mortality rate is 1 in a million injections.27 Gadolinium-containing compounds have been associated with nephrogenic systemic fibrosis (NSF) in patients with renal insufficiency.28
TABLE 32-6. CLINICAL MANIFESTATIONS OF IMMEDIATE AND NONIMMEDIATE HYPERSENSITIVITY REACTIONS TO RADIOCONTRAST AGENTS (MOST FREQUENT ARE UNDERLINED)25
SPECIFIC NONOPERATING ROOM PROCEDURES
Diagnostic and Interventional Radiology
Angiography
Angiography causes minimal discomfort and may be performed under local anesthesia with or without light sedation. Patients are required to remain completely motionless during these procedures, which may be lengthy, particularly spinal angiography. Neurologic disorders such as recent subarachnoid hemorrhage, stroke, and depressed level of consciousness or raised ICP may necessitate anesthesia with formal airway protection. Angiography is usually performed via the femoral artery; the femoral vein may also be accessed when imaging arteriovenous malformations (AVMs) or dural venous abnormalities. Liberal use of local anesthetic at the puncture site precludes the need for intravenous analgesia. The injection of contrast media into the cerebral arteries may cause discomfort, burning, or pruritus around the face and eyes. Hypotension and bradycardia may also occur. During angiography and other interventional radiologic procedures, the patient is placed on a moving gantry and the radiologist positions the patient to track catheters as they pass from the groin into the vessels of interest. It is vital to have extensions on all anesthesia breathing circuits, infusion lines, and monitors to prevent these from being accidentally dislodged as the radiologist swings the X-ray table back and forth. Care should be taken with positioning of radiopaque pieces of equipment. The electrocardiogram electrodes and metallic coils in the cuffs of endotracheal tubes may cause interesting and annoying artifacts if they lie over the area being imaged.
Interventional Neuroradiology
Interventional neuroradiology is an emerging specialty viewed as a hybrid of traditional neurosurgery and neuroradiology and recently defined as the “treatment by endovascular access for the purpose of delivering therapeutic drugs and devices”.29 A variety of neurosurgical conditions especially neurovascular diseases are effectively managed by interventional neuroradiology (Table 32-2).
Cerebral aneurysms and AVMs are particularly amenable to occlusive endovascular treatments. A commonly employed technique is to insert detachable platinum coils into the abnormal vessel(s). Other occlusive agents include cyanoacrylates, “Onyx liquid embolic system” (Microtherapeutics Inc., USA) a biocompatible liquid embolic agent, and polyvinyl alcohol particles. These particles may also be used to produce temporary occlusion of blood vessels for preoperative embolization of vascular tumors such as meningiomas. A large multicenter study, the International Subarachnoid Aneurysm Trial (ISAT), recently reported a better outcome in patients with World Federation of Neurosurgical Societies (WFNS) grades 1 and 2 (good grades) presenting with anterior circulation aneurysms undergoing interventional neuroradiology compared to surgical clipping30–32 In poor WFNS grade cerebral aneurysms and elderly patients, the superiority of one treatment or the other has not been clearly established for these patients.33,34 Interventional neuroradiology is the standard treatment for posterior circulation aneurysms especially basilar artery tip aneurysms.
Procedural and Anesthetic Technique Considerations in Interventional Neuroradiology
For most interventional neuroradiologic procedures, arterial access is gained using a 6 or 7 French gauge sheath via the femoral or, rarely, the carotid or axillary artery. The umbilical vessels are an alternative route in neonates. Anticoagulation is required during and up to 24 hours after interventional radiologic procedures to prevent thromboembolism. Heparin, between 3,000 and 5,000 U (70 IU/kg) followed by an infusion is used to keep the activated clotting time (ACT) between 1.5 and 2.5 times the patient’s baseline.35 General anesthesia and conscious sedation are both suitable techniques for interventional neuroradiology depending on the complexity of the procedure, the need for blood pressure manipulation, and the need for intraprocedural assessment of neurologic function.29,33 General anesthesia is usually conducted with endotracheal intubation and intermittent positive-pressure ventilation, although the laryngeal mask airway (LMA) is a suitable alternative in selected cases.36 Sedation techniques vary; propofol infusions are widely used, as are combinations of a benzodiazepine (usually midazolam) and opioid (usually fentanyl). More recently, dexmedetomidine has been evaluated as a sedative agent that does not cause significant respiratory depression in patients requiring neurologic testing.37 Dexmedetomidine has many advantages as a sedative agent; however, one study demonstrated impairment of cognitive testing in patients undergoing endovascular embolization of cerebral AVMs with dexmedetomidine as the sedative agent.38 The anesthesiologist may facilitate the neuroradiologist in a number of ways by manipulating systemic blood pressure and controlling end-tidal carbon dioxide tension. Controlled hypotension is often requested to facilitate embolization of AVMs; beta-blockers and hydralazine are commonly used. Moderate hypertension may help reduce cerebral ischemia by maintaining cerebral perfusion; in this case, phenylephrine is the agent of choice. Certain procedures require patients to be awake for part of the procedure. The Wada test is used to determine the dominant side for cognitive functions such as speech and memory by injecting sodium amobarbital or methohexital into the carotid artery. This procedure may be used prior to surgery for nonlife-threatening conditions such as epilepsy. The superselective anesthesia functional examination (SAFE), is an extension of the Wada test39 performed prior to therapeutic embolization to ensure that the catheter tip has not been placed in a vessel that supplies an eloquent area of the brain or spinal cord.
Major complications of interventional neuroradiology are hemorrhagic, such as aneurysm rupture, intracranial vessel injury or dissection; occlusive, such as displacement or fragmentation of embolic materials or vasospasm; or non-CNS complications, such as contrast hypersensitivity, anaphylaxis, CIN, and hemorrhage at the peripheral vessel puncture site causing groin or retroperitoneal hematoma.29
Computed Tomography
Computed tomography (CT) scanners obtain a cross-sectional image in a few seconds, and spiral scanners can image a slice of the body in <1 second, minimizing problems with motion artifacts. The procedure is painless and most adults do not require sedation or anesthesia. There is an absolute requirement for the patient to remain motionless while the study is being performed and children or adults with psychological or neurologic disorders preventing immobility may require sedation or anesthesia (Table 32-1). Contrast agents for CT imaging may be administered orally and the anesthesiologist needs to be aware of the possibility of a full stomach. CT scanning may be employed to facilitate invasive procedures such as abscess localization and drainage, ablation of bony metastases, and radiofrequency ablation (RFA) of malignancies. Patients with acute thoracic, abdominal, and cerebral trauma often require urgent imaging to facilitate diagnosis. These patients may develop hemorrhagic shock, raised ICP, depression of consciousness, and cardiac arrest in the CT scanner and must be adequately resuscitated and stabilized before transportation to the radiology department.
Radiofrequency Ablation
Percutaneous RFA is carried out in the radiology suite for treatment of primary and metastatic tumors in the liver, lung, adrenal gland, breast, thyroid, prostate, kidney, and spleen. A high-frequency alternating current is used to generate a localized heat source directly into the tumor causing coagulative necrosis and tumor cell death while avoiding the surrounding tissues. The majority of these procedures are tolerated without sedation. If an anesthesiologist does become involved in the care of these patients, careful evaluation is required; patients may be in the later stages of their disease, have often failed surgical treatment, and may well have undergone extensive radiation therapy and/or chemotherapy. Recently, high frequency jet ventilation (HFJV) has been used for patients undergoing anesthesia for RFA of liver tumors.40 Positioning of the probe is critical and excursions of the diaphragm in ventilated or spontaneously ventilating patients may cause excessive movement; HFJV minimizes liver motion during these procedures.
Transjugular Intrahepatic Portosystemic Shunt
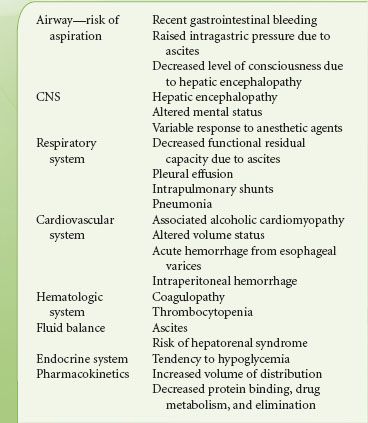
The transjugular intrahepatic portosystemic shunt (TIPS) is created via a catheter inserted in the internal jugular vein and directed into the liver. It connects the right or left portal vein through the liver parenchyma to one of the three hepatic veins.41 The TIPS functions to decompress the portal circulation in patients with portal hypertension and is often performed in patients who have failed to respond to medical therapy.42 The TIPS has been found to be effective in the secondary prophylaxis of bleeding varices and control of refractory cirrhotic ascites.43 It may also be used as a bridge to transplant in patients with poor liver function. The procedure causes minimal stimulation, lasts between 2 and 3 hours, and may be performed under sedation or general anesthesia.44 Patients presenting for a TIPS procedure, in general, have significant hepatic dysfunction, and require careful preoperative assessment and intraoperative management. The considerations are outlined in Table 32-7 (see also Chapter 45).
TABLE 32-7. CONSIDERATIONS IN PATIENTS PRESENTING FOR THE TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT PROCEDURE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree