Neuromuscular blocking agents are used to improve conditions for tracheal intubation, to provide immobility during surgery, and to facilitate mechanical ventilation.
 Anticholinesterases inhibit breakdown of acetylcholine and help restore neuromuscular function after any nondepolarizing blocking agent, but their effect is limited so that their use does not rule out residual paralysis, defined as a train-of-four (TOF) ratio <0.9.
Anticholinesterases inhibit breakdown of acetylcholine and help restore neuromuscular function after any nondepolarizing blocking agent, but their effect is limited so that their use does not rule out residual paralysis, defined as a train-of-four (TOF) ratio <0.9.
 The main site of action of neuromuscular blocking agents (muscle relaxants) is on the nicotinic cholinergic receptor at the endplate of muscle. They also have effects at presynaptic receptors located on the nerve terminal.
The main site of action of neuromuscular blocking agents (muscle relaxants) is on the nicotinic cholinergic receptor at the endplate of muscle. They also have effects at presynaptic receptors located on the nerve terminal.
 The only depolarizing agent still in use is succinylcholine. All other drugs available are nondepolarizing. They compete with acetylcholine for the same binding sites.
The only depolarizing agent still in use is succinylcholine. All other drugs available are nondepolarizing. They compete with acetylcholine for the same binding sites.
 Succinylcholine is a blocking agent that produces depolarization at the endplate and binds to extrajunctional receptors. In spite of many side effects, such as hyperkalemia, its rapid offset makes it the drug of choice for rapid sequence induction.
Succinylcholine is a blocking agent that produces depolarization at the endplate and binds to extrajunctional receptors. In spite of many side effects, such as hyperkalemia, its rapid offset makes it the drug of choice for rapid sequence induction.
 Fade in response to high frequency stimulation (e.g., TOF, 2 Hz for 2 seconds) is a characteristic of nondepolarizing blockade. The TOF fade is difficult to evaluate manually or visually during recovery when TOF ratio is >0.4.
Fade in response to high frequency stimulation (e.g., TOF, 2 Hz for 2 seconds) is a characteristic of nondepolarizing blockade. The TOF fade is difficult to evaluate manually or visually during recovery when TOF ratio is >0.4.
 When using mivacurium, reversal with anticholinesterases should be attempted only when all four twitches in response to TOF stimulation are visible at the adductor pollicis.
When using mivacurium, reversal with anticholinesterases should be attempted only when all four twitches in response to TOF stimulation are visible at the adductor pollicis.
 The upper airway is particularly sensitive to the effects of nondepolarizing blockade. Residual paralysis should be assumed to be present if TOF ratio at the adductor pollicis is <0.9.
The upper airway is particularly sensitive to the effects of nondepolarizing blockade. Residual paralysis should be assumed to be present if TOF ratio at the adductor pollicis is <0.9.
 Neuromuscular transmission can be restored by injection of sugammadex, an agent that binds specifically in a 1:1 molar ratio to rocuronium and vecuronium.
Neuromuscular transmission can be restored by injection of sugammadex, an agent that binds specifically in a 1:1 molar ratio to rocuronium and vecuronium.
 Sugammadex, if given in an appropriate dose, is effective at any depth of blockade.
Sugammadex, if given in an appropriate dose, is effective at any depth of blockade.
Multimedia
 Neuromuscular Blocking Agents
Neuromuscular Blocking Agents
 Fasciculations
Fasciculations
 Monitoring Nerve Block
Monitoring Nerve Block
It appears paradoxical that drugs having peripheral effects on neuromuscular transmission might have a role in anesthesia. If the patient is anesthetized, why provide agents to prevent movement? Yet, the introduction of muscle relaxants, more appropriately called neuromuscular blocking agents, into clinical practice in 1942 was an important milestone in the history of anesthesia.1 While the usefulness of the new drugs became apparent, there were doubts regarding patient safety. In 1954, Beecher and Todd2 claimed that anesthetic mortality increased sixfold when muscle relaxants were used. This situation was probably because of the suboptimal use of mechanical ventilation and reversal drugs, but other controversies have arisen in recent years for a variety  of reasons. Neuromuscular blocking agents are used to improve conditions for tracheal intubation, to provide immobility during surgery, and to facilitate mechanical ventilation.
of reasons. Neuromuscular blocking agents are used to improve conditions for tracheal intubation, to provide immobility during surgery, and to facilitate mechanical ventilation.
For example, the incidence of awareness appears to be greater when neuromuscular blocking agents are used,3 and some authors recommend restricting the use of these drugs whenever possible, as patient movement might be an indicator of consciousness. However, anesthetics act at the spinal cord level to produce immobility; thus, movement in response to a noxious stimulus indicates inadequate analgesia and does not necessarily mean that the patient is conscious.4 Therefore, awareness does not occur because too much neuromuscular blocking agent has been given, but because too little anesthetic is administered. The controversy regarding neuromuscular blocking agents and awareness is complicated by the fact that neuromuscular blockade seems to affect the bispectral index (BIS), which is the most widely used measure of unconsciousness.5 Reductions in BIS have been reported in awake individuals receiving succinylcholine and in mildly sedated patients given mivacurium.
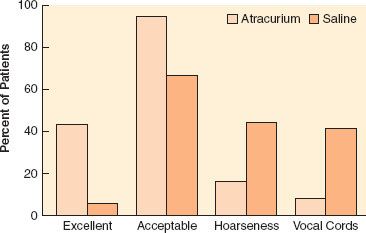
Complete paralysis is not required for the duration of all surgical procedures. However, neuromuscular blocking agents were found to make a difference in lower abdominal surgery, where surgical conditions were better in patients receiving vecuronium (Fig. 20-1).6 In addition to providing immobility and better surgical conditions, neuromuscular blocking agents improve intubating conditions. The doses of opioids required for acceptable intubating conditions in the absence of muscle paralysis produce significant hypotension (Fig. 20-2).7 Providing optimal intubating conditions is not a trivial objective. Poor intubating conditions may increase the incidence of laryngeal injury, as manifested by voice hoarseness and vocal cord damage (Fig. 20-3), and the best way to improve intubating conditions is to administer neuromuscular blocking agents.10
FIGURE 20-1. Surgeon’s assessment of muscle relaxation during lower abdominal surgery. Rating goes from 1 (excellent) to 4 (poor). The incidence of poor rating was greater in patients not given vecuronium (29%) compared with those who received the drug (2%). (Redrawn from: King M, Sujirattanawimol N, Danielson DR, et al. Requirements for muscle relaxants during radical retropubic prostatectomy. Anesthesiology. 2000;93:1392.)
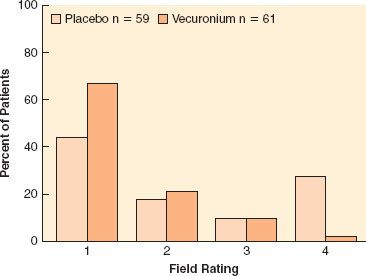
FIGURE 20-2. Neuromuscular blocking agents provide better intubating conditions than high doses of opioids, without hypotension. Hypnotic agent was propofol or thiopental. Intubating conditions are plotted against dose of remifentanil (in micrograms per kilogram). Results for succinylcholine (Sux), 1 mg/kg (with little opioid) are given for comparison. Hypotension was seen with remifentanil, 4 μg/kg. (Data obtained from references: McNeil IA, Culbert B, Russell I. Comparison of intubating conditions following propofol and succinylcholine with propofol and remifentanil 2 micrograms kg–1 or 4 micrograms kg–1. Br J Anaesth. 2000;85:623–625; Naguib M, Samarkandi AH, El-Din ME, et al. The dose of succinylcholine required for excellent endotracheal intubating conditions. Anesth Analg. 2006;102:151–155; Andrews JI, Kumar N, van den Brom RH, et al. A large simple randomized trial of rocuronium versus succinylcholine in rapid-sequence induction of anaesthesia along with propofol. Acta Anaesthesiol Scand. 1999;43:4–8 ; Durmus M, Ender G, Kadir BA, et al. Remifentanil with thiopental for tracheal intubation without muscle relaxants. Anesth Analg. 2003;96:1336; Klemola UM, Mennander S, Saarnivaara L. Tracheal intubation without the use of muscle relaxants: Remifentanil or alfentanil in combination with propofol. Acta Anaesthesiol Scand. 2000;44:465; and Donati F. The right dose of succinylcholine. Anesthesiology. 2003;99:1037)
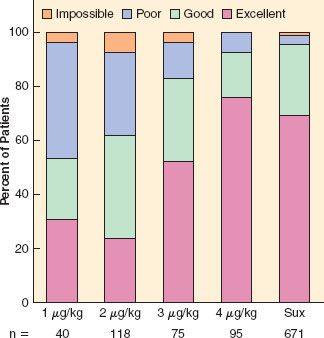
FIGURE 20-3. Neuromuscular blocking agents improve intubating conditions and reduce vocal cord sequelae. The graph depicts the incidence of excellent and acceptable (defined as good or excellent) intubating conditions after atracurium or saline. The percentage of patients who reported hoarseness and those with vocal cord lesions documented by stroboscopy is also shown. (Data from: Mencke T, Echternach M, Kleinschmidt S, et al. Laryngeal morbidity and quality of tracheal intubation: A randomized controlled trial. Anesthesiology. 2003;98:1049–1056.)
The effects of neuromuscular blocking drugs must have worn off or be reversed before the patient regains consciousness. Even with the introduction of shorter-acting neuromuscular blocking agents, reversal of blockade is required in most cases. However, residual paralysis is still a problem, more than 30 years after it was first described (Table 20-1), and in spite of the availability of shorter-acting neuromuscular blocking drugs and widespread use of neuromuscular monitoring.21 Part of this might be related to the recognition that the threshold for complete neuromuscular recovery is a train-of-four (TOF) ratio of 0.9, instead of the  traditional 0.7 (Fig. 20-4).22 Sugammadex, a selective binding agent for the neuromuscular blocking agents rocuronium and vecuronium, was created to address this problem. Thus, an understanding of the pharmacology of neuromuscular blocking agents and reversal drugs is essential.
traditional 0.7 (Fig. 20-4).22 Sugammadex, a selective binding agent for the neuromuscular blocking agents rocuronium and vecuronium, was created to address this problem. Thus, an understanding of the pharmacology of neuromuscular blocking agents and reversal drugs is essential.
FIGURE 20-4. Upper esophageal resting tone in volunteers given vecuronium. Train-of-four (TOF) ratio was measured at the adductor pollicis muscle. Statistically significant decreases compared with control were found at all levels of paralysis until TOF ratio >0.9. (Redrawn from: Eriksson LI, Sundman E, Olsson R, et al. Functional assessment of the pharynx at rest and during swallowing in partially paralyzed humans: Simultaneous videomanometry and mechanomyography of awake human volunteers. Anesthesiology. 1997;87:1035.)
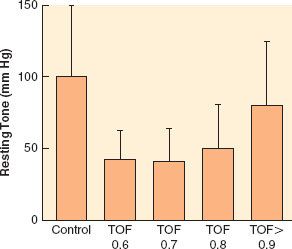
TABLE 20-1. SELECTED REPORTS OF RESIDUAL PARALYSIS 1979–2011
PHYSIOLOGY AND PHARMACOLOGY
Structure
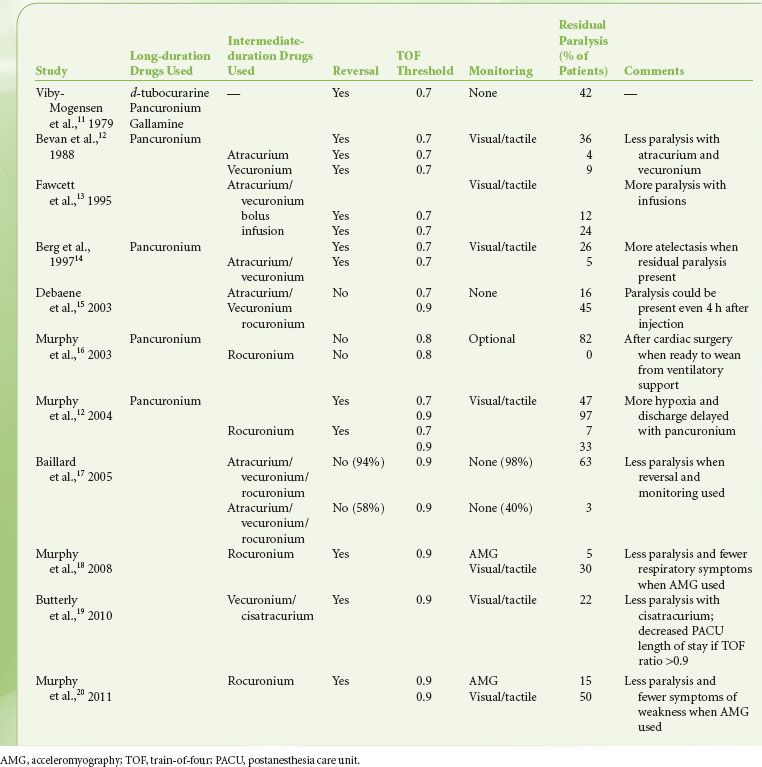
The cell bodies of motor neurons supplying skeletal muscle lie in the spinal cord. They receive and integrate information from the central nervous system. This information is carried via an elongated structure, the axon, to distant parts of the body. Each nerve cell supplies many muscle cells (or fibers) a short distance after branching into nerve terminals. The terminal portion of the axon is a specialized structure, the synapse, designed for the production and release of acetylcholine. The synapse is separated from the  endplate of the muscle fiber by a narrow gap, called the synaptic cleft, which is approximately 50 nm in width (0.05 μm) (Fig. 20-5).23–25 The nerve terminal is surrounded by a Schwann cell, and the synaptic cleft has a basement membrane and contains filaments that anchor the nerve terminal to the muscle.
endplate of the muscle fiber by a narrow gap, called the synaptic cleft, which is approximately 50 nm in width (0.05 μm) (Fig. 20-5).23–25 The nerve terminal is surrounded by a Schwann cell, and the synaptic cleft has a basement membrane and contains filaments that anchor the nerve terminal to the muscle.
FIGURE 20-5. Schematic representation of the neuromuscular junction (not drawn to scale).
The main site of action of neuromuscular blocking agents (muscle relaxants) is on the nicotinic cholinergic receptor at the endplate of the muscle. They also have effects at presynaptic receptors located on the nerve terminal. The endplate is a specialized portion of the membrane of the muscle fiber where nicotinic acetylcholine receptors are concentrated. During development, multiple connections are made between nerve terminals and a single muscle fiber. However, as maturation continues, most of these connections atrophy and disappear, usually leaving only one connection per muscle fiber. This endplate continues to differentiate from the rest of the muscle fiber. The nerve terminal enlarges, and folds appear. The acetylcholine receptors cluster at the endplate, especially at the crests of the folds, and their density decreases to almost zero in extrajunctional areas.23,25 Initially, the receptor density is of the order of 1,000/μm2 of membrane surface. After maturation of the endplate, junctional receptor density increases to 10,000/μm2 or more. Mammalian endplates usually have an oval shape with the short axis perpendicular to the fiber; their surface area is approximately 1,000 μm2. The width of the endplate is sometimes as large as the diameter of the fiber, but is usually smaller. However, its length is only a small fraction of that of the fiber.
Nerve Stimulation
Under resting conditions, the electrical potential of the inside of a nerve cell is negative with respect to the outside (typically –90 mV). If this potential is made less negative (depolarization), sodium channels open and allow sodium ions to enter the cell. This influx of positive ions makes the potential inside the membrane positive with respect to the outside. This potential change, in turn, causes depolarization of the next segment of membrane, causing more sodium channels to open, and an electrical impulse, or action potential, propagates. The duration of the action potential is brief (<1 ms) because of rapid inactivation of sodium channels and activation of potassium channels. An action potential also triggers the opening of calcium channels, allowing calcium ions to penetrate the cell. This entry of calcium facilitates release of the neurotransmitter at the nerve terminal.
The sodium channels in the axon may be activated in response to electrical depolarization provided by a nerve stimulator. A peripheral nerve is made up of a large number of axons, each of which responds in an all-or-none fashion to the stimulus applied. Thus, in the absence of neuromuscular blocking agents, the relationship between the amplitude of the muscle contraction and current applied is sigmoid. At low currents, the depolarization is insufficient in all axons. As current increases, more and more axons are depolarized to threshold and the strength of the muscle contraction increases. When the stimulating current reaches a certain level, all axons are depolarized to threshold and propagate an action potential. Increasing current beyond this point does not increase the amplitude of muscle contraction: The stimulation is supramaximal (Fig. 20-6). Most commercially available stimulators deliver impulses lasting 0.1 to 0.2 ms.
FIGURE 20-6. Example of increasing stimulating current in one patient. Current pulses, 0.2 ms duration, were delivered to the ulnar nerve at the wrist every 10 seconds. The force of contraction of the adductor pollicis muscle was measured and appears as spikes. No twitch was seen if the current was <28 mA. At current strengths of ≥40 mA, the current became supramaximal; increasing the current produced little change in force.
Release of Acetylcholine
Acetylcholine is synthesized from choline and acetate and packaged into 45 nm vesicles. Each vesicle contains 5,000 to 10,000 acetylcholine molecules. Some of these vesicles cluster near the cell membrane opposite the crests of the junctional folds of the endplate, in areas called active zones (Fig. 20-5).23,24
It is now widely accepted that acetylcholine is released in packets, or quanta, and that a quantum represents the contents of one vesicle. In the absence of nerve stimulation, quanta are released spontaneously, at random, and this is seen as small depolarizations of the endplate (miniature endplate potential). When an action potential invades the nerve terminal, approximately 200 to 400 quanta are released simultaneously, unloading approximately 1 to 4 million acetylcholine molecules into the synaptic cleft.23,25 Calcium, which enters the nerve terminal through channels that open in response to depolarization, is required for vesicle fusion and release. Calcium channels are located near docking proteins, and this special geometric arrangement provides high intracellular concentrations of calcium to allow binding of specialized proteins on the vesicle membrane with docking proteins.23,24 Binding produces fusion of the membranes and release of acetylcholine ensues. When the calcium concentration is decreased, or if the action of calcium is antagonized by magnesium, the release process is inhibited and transmission failure may occur. Other proteins regulate storage and mobilization of acetylcholine vesicles. It appears that a small proportion of vesicles is immediately releasable, while a much larger reserve pool can be mobilized more slowly. Each impulse releases 0.2% to 0.5% of the 75,000 to 100,000 vesicles in the nerve terminal. With repetitive stimulation, the amount of acetylcholine released decreases rapidly because only a small fraction of the vesicles is in a position to be released immediately. To sustain release during high-frequency stimulation, vesicles must be mobilized from the reserve pool.
Postsynaptic Events
The receptors at the endplate are of the nicotinic cholinergic type. They are members of the class of Cys-loop ligand-gated ion channels, to which glycine, 5-HT3, and GABA receptors belong.26 These receptors are made up of five glycoprotein subunits arranged in the form of a rosette and lying across the whole cell membrane (Fig. 20-7). To date, 17 subtypes of the nicotinic receptor have been identified, and they differ by the type and arrangement of the subunits.23 The nicotinic subtype present at the neuromuscular junction is made up of two identical subunits, designated α, and three others, called β, δ, and ∊. There are two acetylcholine binding sites, each located on the outside part of the α subunit. When two acetylcholine molecules bind simultaneously to each binding site, an opening is created in the center of the rosette, allowing sodium ions to enter the cell and potassium ions to exit.23,24 The inward movement of sodium is predominant because it is attracted by the negative voltage of the inside of the cell. This movement of sodium depolarizes the endplate; that is, its inside becomes less negative. There is a high density of sodium channels in the folds of synaptic clefts and in the perijunctional area.24,25 These channels open when the membrane is depolarized beyond a critical point, allowing more sodium to enter the cell and producing further depolarization. This depolarization generates an action potential, which propagates by activation of sodium channels along the whole length of the muscle fiber. The muscle action potential has a duration of 5 to 15 ms and can be recorded as an electromyogram (EMG). It precedes the onset of contraction, or twitch, which lasts 100 to 200 ms. With high frequency (>10 Hz) stimulation, the muscle fiber does not have time to relax before the next impulse, so contractions fuse and add up, and a tetanus is obtained.
FIGURE 20-7. Four types of nicotinic receptors involved in neuromuscular transmission. All have five subunits. Postsynaptic adult receptors at the endplate have two α1, one β1, one δ, and one ∊ subunit. The immature, fetal or extrajunctional receptor has the same structure, except for a substitution of the ∊ by the γ subunits. It is distributed throughout the muscle membrane. Receptors made up of only α7 subunits are also found in muscle. Presynaptic receptors, found in the nerve terminal are thought to be of the α3β2 type. The acetylcholine binding sites are represented by a shaded oval area.
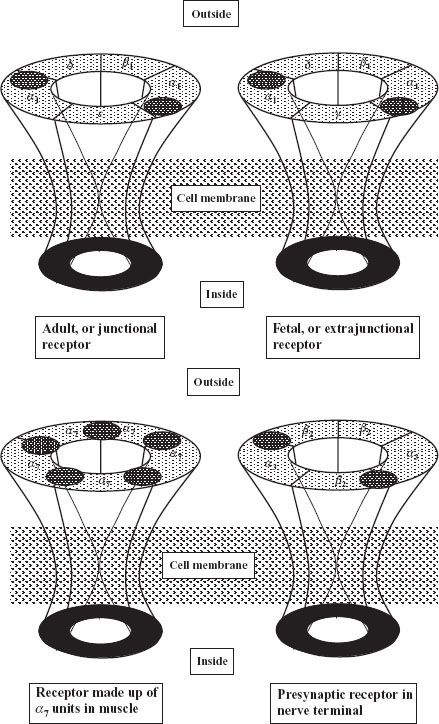
Early in development, receptors are slightly different from those found at the adult endplate: They have a γ subunit instead of an ∊ subunit (Fig. 20-7), and they tend to be evenly distributed along the whole length of the muscle fiber.23–25 The receptors with a γ subunit, are called fetal receptors. When the endplate develops, receptors tend to cluster at the neuromuscular junction and the density is low (typically 10/μm2) in the extrajunctional areas.25 As maturation continues, the γ subunit is substituted by an ∊ subunit, which is characteristic of the adult type, junctional receptor. In humans, the switch occurs in the third trimester of pregnancy. During development, neuronal α7 receptors are also found in the muscle membrane. They are called neuronal because they are also found in the central nervous system, and they are made up of five identical α subunits, which differ slightly from the α subunits of the receptor at the neuromuscular junction, which are called α1. Maintenance of adult receptors at the endplate depends on the integrity of nerve supply. A few γ-type and α7 extrajunctional receptors still persist in adults and can proliferate in cases of denervation.23
The main action of nondepolarizing neuromuscular blocking drugs is to bind to at least one of the two α subunits of the postsynaptic receptor. This prevents access to the receptor by acetylcholine and does not produce opening of the receptor. Under normal circumstances, only a small fraction of available receptors must bind to acetylcholine to produce sufficient depolarization to trigger a muscle contraction. In other words, there is a wide “margin of safety”.27 This redundancy implies that neuromuscular blocking drugs must be bound to a large number of receptors before any blockade is detectable. Animal studies suggest that 75% of receptors must be occupied before twitch height decreases in the presence of d-tubocurarine, and blockade is complete when 92% of receptors are occupied. The actual number depends on species and type of muscle, and humans might have a reduced margin of safety compared with other species.27 So it is futile to correlate receptor occupancy data obtained in cats with certain clinical tests in humans, such as handgrip and head lift, which involve different muscle groups. However, the general concept that a large proportion of receptors must be occupied before blockade becomes detectable, and that measurable blockade occurs over a narrow range of receptor occupancy, remains applicable to clinical practice. Because it must overcome the margin of safety, the initial dose of neuromuscular blocking agent is greater than maintenance doses.
Acetylcholine is hydrolyzed rapidly by the enzyme acetyl cholinesterase, which is present in the folds of the endplate. It is also attached to stalks attached to the basement membrane in the synaptic cleft. The presence of the enzyme in the synaptic cleft suggests that not all the acetylcholine released reaches the endplate; some is hydrolyzed en route.25,28
Presynaptic Events
The release of acetylcholine normally decreases during high frequency stimulation because the pool of readily releasable acetylcholine becomes depleted faster than it can be replenished. Under normal circumstances, the reduced amount released is well above what is required to produce muscle contraction because of the high margin of safety at the neuromuscular junction. In addition, a positive feedback system involving activation of presynaptic receptors helps in the mobilization of acetylcholine vesicles.23 There is some evidence that the presynaptic and postsynaptic receptors are of different subtypes, and that presynaptic receptors are most likely of the α3β2 subtype, that is, they are made up of only α and β subunits.29 This receptor contains α subunits that are slightly different from those found in postsynaptic receptors, thus the designation as α3, instead of the α1, and the β subunits (β2) are also slightly different from the β1 subunit found in postsynaptic receptors.
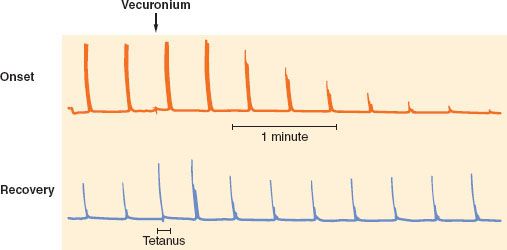
The putative role of the presynaptic receptors is to maintain the number of vesicles ready to be released. Nondepolarizing neuromuscular blocking drugs produce characteristic TOF and tetanic fade, probably by blocking presynaptic nicotinic receptors,23,29 thus preventing mobilization of acetylcholine vesicles and leading to reduced acetylcholine release during high frequency stimulation. Succinylcholine has virtually no effect on these presynaptic receptors, which would explain the lack of fade observed with this drug. Presynaptic receptors may not be the only elements contributing to the fade phenomenon: They do not explain why fade is more prominent during onset than during recovery (Fig. 20-8), and why after sugammadex, TOF ratio is restored faster than first twitch.30 Fade constitutes a key property of nondepolarizing neuromuscular blocking drugs and is useful for monitoring purposes.
FIGURE 20-8. Characteristics of nondepolarizing blockade. Train-of-four (TOF) responses are equal before administration of vecuronium (arrow). For a given twitch depression, fade is less during onset (top trace) than recovery (bottom trace). A 50 Hz tetanus was applied during recovery, and the response is not sustained (tetanic fade). The TOF response immediately after the tetanus is enhanced, and progressively returns to its normal value within 1 to 2 minutes.
NEUROMUSCULAR BLOCKING AGENTS
Neuromuscular blocking drugs interact with the acetylcholine receptor either by depolarizing the endplate (depolarizing agents) or by competing with acetylcholine for binding sites (nondepolarizing agents). The only depolarizing agent still in use is  succinylcholine. All others are of the nondepolarizing type.
succinylcholine. All others are of the nondepolarizing type.
Pharmacologic Characteristics of Neuromuscular Blocking Agents
The effect of neuromuscular blocking drugs is measured as the depression of adductor muscle contraction (twitch) following electrical stimulation of the ulnar nerve. The value is compared with a control value, obtained before injection of the drug. Each drug has characteristic onset, potency, duration of action, and recovery index.
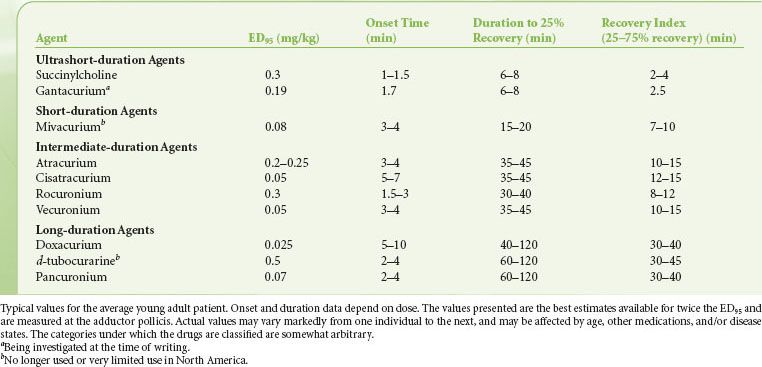
Potency of a drug is determined by constructing dose-response curves, which describe the relationship between twitch depression and dose (Fig. 20-9).31 Then, the effective dose 50, or ED50, which is the median dose corresponding to 50% twitch depression, is obtained. Because clinically useful relaxation is attained when twitch is abolished almost completely, the ED95, corresponding to 95% block, is more commonly used. For example, the ED95 for vecuronium is 0.05 mg/kg, which means that half the patients will achieve at least 95% block of single twitch (compared with the pre-vecuronium value) with that dose, and half the subjects will reach <95% block. Rocuronium has an ED95 of 0.3 mg/kg. Therefore, it has one-sixth the potency of vecuronium. In other words, compared with vecuronium, six times as much rocuronium has to be given to produce the same effect. The ED95 of known neuromuscular blocking agents vary over two orders of magnitude (Table 20-2).
FIGURE 20-9. Example of a dose-response relationship. The actual numbers are approximately those for rocuronium. The ED50 is the dose corresponding to 50% blockade and ED95 is the dose corresponding to 95% blockade.
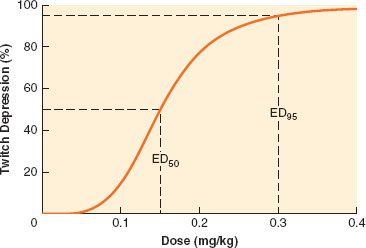
TABLE 20-2. POTENCY, ONSET TIME, DURATION, AND RECOVERY INDEX OF SELECTED NEUROMUSCULAR BLOCKING AGENTS
Onset time, or time to maximum blockade, can be shortened if the dose is increased. When two or more drugs are compared, it is meaningful to compare only equipotent doses and usually clinically relevant doses (2 × ED95) are considered.31
Duration of action is the time from injection of the neuromuscular blocking agent to return of 25% twitch height (compared with control). Duration increases with dose, so it is usual to make comparisons between 2 × ED95 doses, which are normal intubation doses. The 25% twitch height figure was chosen because rapid reversal can normally be achieved at that level. For nondepolarizing agents, the fourth twitch usually reappears when first twitch is close to 25%. Categories were proposed for neuromuscular blocking drugs according to their duration of action (Table 20-2).31 Agents with the same duration may have markedly different onsets.
Recovery index is the time interval between 25% and 75% twitch height. It provides information about the speed of recovery once return of twitch is manifest. Contrary to duration of action, it does not depend heavily on the dose given. The values for ED95, onset time, duration of action, and recovery index depend on which muscle is used to make measurements. For consistency, the adductor pollicis muscle has been retained as the gold standard, not because of its physiologic significance but because it is most commonly monitored and data on it are most abundant.
The pharmacologic characteristics of neuromuscular blocking agents are completed by an assessment of intubating conditions, which do not always parallel twitch height at the adductor pollicis muscle. Intubating conditions depend on paralysis of centrally located muscles, but also on the type and quantity of opioid and hypnotic drugs given for induction of anesthesia. To decrease variability between studies, criteria to grade intubating conditions as excellent, good, poor, or impossible in a scoring system were adopted by a group of experts who met in Copenhagen in 1994, and a revision of these standards was adopted at a later meeting in Stockholm in 2005.32
DEPOLARIZING DRUGS: SUCCINYLCHOLINE
 Among drugs that depolarize the endplate, only succinylcholine is still used clinically. In spite of a long list of undesired effects, succinylcholine remains popular because it is the only ultrarapid onset/ultrashort duration neuromuscular blocking drug currently available.
Among drugs that depolarize the endplate, only succinylcholine is still used clinically. In spite of a long list of undesired effects, succinylcholine remains popular because it is the only ultrarapid onset/ultrashort duration neuromuscular blocking drug currently available.
Neuromuscular Effects
The effects of succinylcholine at the neuromuscular junction are not completely understood. The drug depolarizes postsynaptic and extrajunctional receptors. However, when the receptor is in contact with any agonist, including acetylcholine, for longer than a few milliseconds it ceases to respond to the agonist. Normally, this desensitization process does not occur with acetylcholine because of its rapid breakdown (<1 ms). However, succinylcholine remains at the endplate for much longer, so desensitization develops after a brief period of activation.33 Another possible mechanism is the inactivation of sodium channels in the junctional and perijunctional areas, which occurs when the membrane remains depolarized.33 This inactivation prevents the propagation of the action potential. Both desensitization of the receptor and inactivation of sodium channels might be present together.
 Within 1 minute after succinylcholine injection and before paralysis is manifest, disorganized movements, or fasciculations,
Within 1 minute after succinylcholine injection and before paralysis is manifest, disorganized movements, or fasciculations,  are frequently observed. This activity probably reflects the agonist effect of succinylcholine, before desensitization takes place. Small doses of nondepolarizing drugs are effective in reducing the incidence of fasciculations.34
are frequently observed. This activity probably reflects the agonist effect of succinylcholine, before desensitization takes place. Small doses of nondepolarizing drugs are effective in reducing the incidence of fasciculations.34
Succinylcholine has yet another neuromuscular effect. In some muscles, like the masseter and to a lesser extent the adductor pollicis, a sustained increase in tension that may last for several minutes can be observed. The mechanism of action of this tension change is uncertain but is most likely mediated by acetylcholine receptors because it is blocked by large amounts of nondepolarizing drugs.35 The increase in masseteric tone, which is probably always present to some degree but is greater in some susceptible individuals, may lead to imperfect intubating conditions in a small proportion of patients. Masseter muscle spasm may be an exaggerated form of this response.
Characteristics of Depolarizing Blockade
After injection of succinylcholine, single-twitch height is decreased. However, the response to high frequency stimulation is sustained: Minimal TOF and tetanic fade is observed. The block is antagonized by nondepolarizing agents so that the ED95 is doubled if a small “defasciculating” dose of nondepolarizing drug is given prior to the intubating dose of succinylcholine, thus requiring an increased dose of succinylcholine.36 Succinylcholine blockade is potentiated by inhibitors of acetyl cholinesterase, such as neostigmine and edrophonium. After administration of 7 to 10 mg/kg, or 30 to 60 minutes of exposure to succinylcholine, TOF and tetanic fade become apparent. Neostigmine or edrophonium can antagonize this block, which has been termed nondepolarizing, dual, or phase II block. The onset of phase II block coincides with tachyphylaxis, as more succinylcholine is required for the same effect.
Pharmacology of Succinylcholine
Succinylcholine is rapidly hydrolyzed by plasma cholinesterase (also called pseudocholinesterase), with an elimination half-life of <1 minute in patients.37 Because of the rapid disappearance of succinylcholine from plasma, the maximum effect is reached quickly. Sub-paralyzing doses (up to 0.3 to 0.5 mg/kg) reach their maximal effect within approximately 1.5 to 2 minutes at the adductor pollicis muscle,36 and within 1 minute at more central muscles, such as the masseter, the diaphragm, and the laryngeal muscles. With larger doses (1 to 2 mg/kg), abolition of twitch response can be reached even more rapidly (<1 minute).
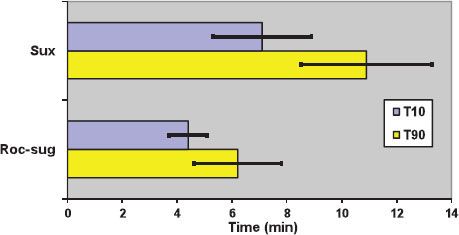
The mean dose producing 95% blockade (ED95) at the adductor pollicis muscle is 0.30 to 0.50 mg/kg.36 These values are doubled if d-tubocurarine, 0.05 mg/kg, is given as a defasciculating agent.36 The time until full recovery at the adductor pollicis is dose-dependent and reaches 10 to 12 minutes after a dose of 1 mg/kg (Fig. 20-10).38 The diaphragm starts contracting and spontaneous breathing resumes after approximately 5 minutes.39
FIGURE 20-10. Duration of action of neuromuscular blockade with succinylcholine 1 mg/kg (Sux), and rocuronium 1.2 mg/kg followed 3 minutes later by sugammadex 16 mg/kg (Roc-sug). T10 and T90: Duration to 10% and 90% first twitch recovery, respectively. Bars indicate standard deviation. Data from: Lee C, Jahr JS, Candiotti KA, et al. Reversal of profound neuromuscular block by sugammadex administered three minutes after rocuronium. Anesthesiology. 2009;110:1020–1025.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






