Anesthesiologists routinely manage patients during their perioperative medical care where they are exposed to multiple agents that can produce an allergic response including drugs (antibiotics, anesthetic agents, neuromuscular blocking agents [NMBAs], sedative-hypnotics), polypeptides (i.e., protamine), blood products, and environmental antigens (i.e., latex).
 Antibodies are specific proteins called immunoglobulins that can recognize and bind to a specific antigen, and usually IgE or IgG is implicated.
Antibodies are specific proteins called immunoglobulins that can recognize and bind to a specific antigen, and usually IgE or IgG is implicated.
 Cytokines are inflammatory cell activators that are synthesized to act as secondary messengers and activate endothelial cells and white cells.
Cytokines are inflammatory cell activators that are synthesized to act as secondary messengers and activate endothelial cells and white cells.
 Immune competence during surgery can be affected by direct and hormonal effects of anesthetic drugs, by immunologic effects of other drugs used, by the surgery, by coincident infection, and by transfused blood products.
Immune competence during surgery can be affected by direct and hormonal effects of anesthetic drugs, by immunologic effects of other drugs used, by the surgery, by coincident infection, and by transfused blood products.
 Most of the allergic reactions evoked by intravenous drugs occur within 5 minutes of administration. In the anesthetized patient, the most common life-threatening manifestation of an allergic reaction is circulatory collapse, reflecting vasodilation with resulting decreased venous return.
Most of the allergic reactions evoked by intravenous drugs occur within 5 minutes of administration. In the anesthetized patient, the most common life-threatening manifestation of an allergic reaction is circulatory collapse, reflecting vasodilation with resulting decreased venous return.
 Many diverse molecules administered during the perioperative period release histamine in a dose-dependent, nonimmunologic fashion.
Many diverse molecules administered during the perioperative period release histamine in a dose-dependent, nonimmunologic fashion.
 A plan for treating anaphylactic reactions must be established before the event. Airway maintenance, 100% oxygen administration, intravascular volume expansion, and epinephrine are essential to treat the hypotension and hypoxia that result from vasodilation, increased capillary permeability, and bronchospasm. Vasopressin and additional diagnostic monitoring should be considered for refractory shock.
A plan for treating anaphylactic reactions must be established before the event. Airway maintenance, 100% oxygen administration, intravascular volume expansion, and epinephrine are essential to treat the hypotension and hypoxia that result from vasodilation, increased capillary permeability, and bronchospasm. Vasopressin and additional diagnostic monitoring should be considered for refractory shock.
 After an anaphylactic reaction, it is important to attempt to identify the causative agent to prevent readministration.
After an anaphylactic reaction, it is important to attempt to identify the causative agent to prevent readministration.
 Health-care workers and children with spina bifida, urogenital abnormalities, or certain food allergies have been recognized as people at increased risk for anaphylaxis to latex.
Health-care workers and children with spina bifida, urogenital abnormalities, or certain food allergies have been recognized as people at increased risk for anaphylaxis to latex.
 NMBAs have several unique molecular features that make them potential antigens.
NMBAs have several unique molecular features that make them potential antigens.
Multimedia
 Type 3 Immune Complex Reaction
Type 3 Immune Complex Reaction
 Anaphylaxis
Anaphylaxis
INTRODUCTION
Allergic reactions represent an important cause of perioperative complications.  Anesthesiologists routinely administer multiple drugs and blood products and manage patients during their perioperative medical care where they are exposed to multiple agents including drugs (i.e., antibiotics, anesthetic agents, neuromuscular blocking agents [NMBAs], sedative-hypnotics), polypeptides (protamine, aprotinin), blood products, and environmental antigens (i.e., latex). Anesthesiologists must be able to rapidly recognize and treat anaphylaxis, the most life-threatening form of an allergic reaction.1
Anesthesiologists routinely administer multiple drugs and blood products and manage patients during their perioperative medical care where they are exposed to multiple agents including drugs (i.e., antibiotics, anesthetic agents, neuromuscular blocking agents [NMBAs], sedative-hypnotics), polypeptides (protamine, aprotinin), blood products, and environmental antigens (i.e., latex). Anesthesiologists must be able to rapidly recognize and treat anaphylaxis, the most life-threatening form of an allergic reaction.1
The allergic response represents just one aspect of the pathologic response that the immune system evolved to recognize foreign substances. As part of normal host surveillance mechanisms, a series of cellular and humoral elements oversees foreign surfaces of cell surfaces and molecular structures called antigens to provide host defense. These foreign substances (antigens) consist of molecular arrangements found on cells, bacteria, viruses, proteins, or complex macromolecules.2,3 Immunologic mechanisms (1) involve antigen interaction with antibodies or specific effector cells; (2) are reproducible; and (3) are specific and adaptive, distinguishing foreign substances and amplifying reactivity through a series of inflammatory cells and proteins. The immune system serves to protect the body against external microorganisms and toxins, as well as internal threats from neoplastic cells; however, it can respond inappropriately to cause hypersensitive (allergic) reactions. Life-threatening allergic reactions to drugs and other foreign substances observed perioperatively may represent different expressions of the immune response.2,3
BASIC IMMUNOLOGIC PRINCIPLES
The immune system protects the body from invasion by organisms by recognizing and removing foreign substances called antigens that are molecular structures, usually proteins and/or carbohydrates. However, the body also has mechanisms to tolerate similar molecular configurations of the host (self- tolerance); however, problems arise when the immune system is dysfunctional as in cases of autoimmunity that can give rise to serious diseases including rheumatoid arthritis and lupus. The immune response includes both cell-mediated immunity and humoral immunity. Cell-mediated immunity involves immune cell directed at elimination or destruction of pathogens or cells. Humoral immunity comprises different antibodies and proteins such as complement that can directly or in concert with cellular immunity orchestrate cell injury and destruction. The purpose is to provide host defense mechanisms.
As part of humoral immune responses, protein mediators called cytokines and chemokines are released initially by inflammatory responses to bring other immune cells to the site of the injury or infection, and cause further inflammatory responses and fever, and to increase capillary permeability to allow other immune cells to migrate and translocate to the site of injury. This inflammatory response also causes hemostatic activation and produces pain, erythema, and edema locally and potentially systemically depending on the extent of the injury. Cytokines have an extensive spectrum of inflammatory effects, an issue studied more extensively in sepsis.4 The immune response can be variable in onset from immediate in anaphylaxis, to days, and can remember antigens for many years, especially following immunization.
Although this is a simplified review of the immune system, it is important to consider individual aspects of the immune response and their importance below.
TABLE 12-1. AGENTS ADMINISTERED DURING ANESTHESIA THAT ACT AS ANTIGENS
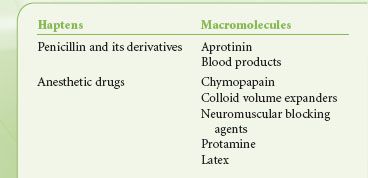
Antigens
As mentioned, molecules stimulating an immune response (antibody production or lymphocyte stimulation) are called antigens.5,6 Only a few drugs used by anesthesiologists, such as polypeptides (protamine) and other large macromolecules (dextrans), are complete antigens (Table 12-1). Most commonly used drugs are simple organic compounds of low molecular weight (around 1,000 Da). For such a small molecule to become immunogenic, it must form a stable bond with circulating proteins or tissue micromolecules to result in an antigen (hapten-macromolecular complex). Small molecular weight substances such as drugs or drug metabolites that bind to host proteins or cell membranes to sensitize patients are called haptens. Haptens are not antigenic by themselves. Often, a reactive drug metabolite (i.e., penicilloyl derivative of penicillin) is believed to bind with macromolecules to become antigens, but for most drugs this has not been proved. Some molecular structures in bacteria or fungi are immediately recognized as foreign.
Thymus-derived (T-cell) and Bursa-derived (B-cell) Lymphocytes
The thymus of the fetus differentiates immature lymphocytes into thymus-derived cells (T cells). T cells have receptors that are activated by binding with foreign antigens and secrete mediators that regulate the immune response. The subpopulations of T cells that exist in humans include helper, suppressor, cytotoxic, and killer cells.5,6 The two types of regulatory T cells are helper cells (OKT4) and suppressor cells (OKT8). Helper cells are important for key effector cell responses, whereas suppressor cells inhibit immune function. Infection of helper T cells with a retrovirus, the human immunodeficiency virus, produces a specific increase in the number of suppressor cells. Cytotoxic T cells destroy mycobacteria, fungi, and viruses. Other lymphocytes, called natural killer cells, do not need specific antigen stimulation to set up their role. Both the cytotoxic T cells and natural killer cells take part in defense against tumor cells and in transplant rejection. T cells produce mediators that influence the response of other cell types involved in the recognition and destruction of foreign substances.5,6
B cells represent a specific lymphocyte cell line that can differentiate into specific plasma cells that synthesize antibodies, a step controlled by both helper and suppressor T-cell lymphocytes. B cells are also called bursa-derived cells because in birds, the bursa of Fabricius is important in producing cells responsible for antibody synthesis.
Antibodies
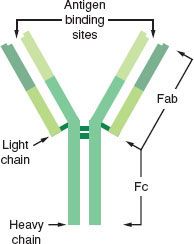
Antibodies are  specific proteins called immunoglobulins (Igs) that can recognize and bind to a specific antigen. The basic structure of the antibody molecule is illustrated in Figure 12-1. Each antibody has at least two heavy chains and two light chains that are bound together by disulfide bonds. The Fab fragment has the ability to bind antigen, and the Fc, or crystallizable, fragment is responsible for the unique biologic properties of the different classes of Igs (cell binding and complement activation). Antibodies function as specific receptor molecules for immune cells and proteins. When antigen binds covalently to the Fab fragments, the antibody undergoes conformational changes to activate the Fc receptor. The results of antigen–antibody binding depend on the cell type, which causes a specific type of activation (i.e., lymphocyte proliferation and differentiation into antibody-secreting cells, mast cell degranulation, and complement activation).
specific proteins called immunoglobulins (Igs) that can recognize and bind to a specific antigen. The basic structure of the antibody molecule is illustrated in Figure 12-1. Each antibody has at least two heavy chains and two light chains that are bound together by disulfide bonds. The Fab fragment has the ability to bind antigen, and the Fc, or crystallizable, fragment is responsible for the unique biologic properties of the different classes of Igs (cell binding and complement activation). Antibodies function as specific receptor molecules for immune cells and proteins. When antigen binds covalently to the Fab fragments, the antibody undergoes conformational changes to activate the Fc receptor. The results of antigen–antibody binding depend on the cell type, which causes a specific type of activation (i.e., lymphocyte proliferation and differentiation into antibody-secreting cells, mast cell degranulation, and complement activation).
FIGURE 12-1. Basic structural configuration of the antibody molecule representing human immunoglobulin G (IgG). Immunoglobulins are composed of two heavy chains and two light chains bound by disulfide linkages (represented by crossbars). Papain cleaves the molecule into two Fab fragments and one Fc fragment. Antigen binding occurs on the Fab fragments, whereas the Fc segment is responsible for membrane binding or complement activation. (Reprinted with permission from: Levy JH. Anaphylactic Reactions in Anesthesia and Intensive Care. 2nd ed. Boston, MA: Butterworth-Heinemann; 1992.)
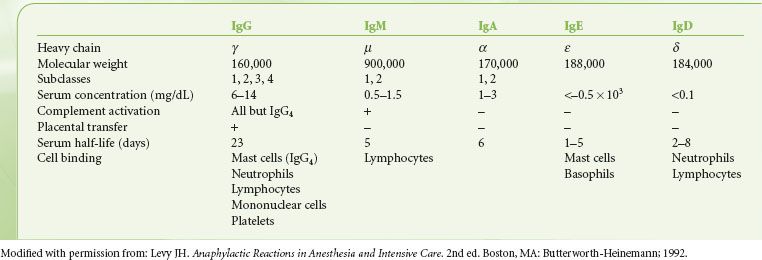
Five major classes of antibodies occur in humans: IgG, IgA, IgM, IgD, and IgE. The heavy chain determines the structure and the function of each molecule. The basic properties of each antibody are listed in Table 12-2.
TABLE 12-2. BIOLOGIC CHARACTERISTICS OF IMMUNOGLOBULINS
Effector Cells and Proteins of the Immune Response
Cells
Monocytes, neutrophils (polymorphonuclear leukocytes [PMNs]), and eosinophils represent important effector cells that migrate into areas of inflammation in response to specific chemotactic factors, including lymphokines, cytokines, and complement-derived mediators. The deposition of antibody or complement fragments on the surface of foreign cells is called opsonization, a process that promotes killing foreign cells by effector cells. In addition, lymphokines and cytokines produce chemotaxis of other inflammatory cells in a manner described in the following sections.5,6 Activation of these cellular process is orchestrated by multiple mechanisms, as best recently reviewed.7
Monocytes and Macrophages
Macrophages regulate immune responses by processing and presenting antigens to effect inflammatory, tumoricidal, and microbicidal functions. Macrophages arise from circulating monocytes or may be confined to specific organs such as the lung. They are recruited and activated in response to microorganisms or tissue injury. Macrophages ingest antigens before they interact with receptors on the lymphocyte surface to regulate their action. Macrophages synthesize mediators to facilitate both B-lymphocyte and T-lymphocyte responses.
Polymorphonuclear Leukocytes (Neutrophils)
The first cells to appear in acute inflammatory reaction are neutrophils that contain acid hydrolases, neutral proteases, and lysosomes. Once activated, they produce hydroxyl radicals, superoxide, and hydrogen peroxide, which aid in microbial killing.
Eosinophils
The exact function of the eosinophil in host defense is unclear; however, inflammatory cells recruit eosinophils to collect at sites of parasitic infections, tumors, and allergic reactions.
Basophils
Basophils comprise 0.5% to 1% of circulating granulocytes in the blood. On the surface of basophils are IgE receptors, which function similarly to those on mast cells.
Mast Cells
Mast cells are important cells for immediate hypersensitivity responses. They are tissue fixed and located in the perivascular spaces of the skin, lung, and intestine. On the surface of mast cells are IgE receptors, which bind to specific antigens. Once activated, these cells release physiologically active mediators important to immediate hypersensitivity responses (see IgE-mediated Pathophysiology section under Anaphylactic Reactions). Mast cells can be activated by a series of both immune and nonimmune stimuli.
Proteins
Cytokines/Interleukins
Cytokines are inflammatory  cell activators that are synthesized by macrophages to act as secondary messengers and activate endothelial cells and white cells.8 Interleukin-1 and tumor necrosis factor are examples of cytokines considered to be important mediators of the biologic responses to infection and other inflammatory reactions. Liberation of interleukin-1 and tumor necrosis factor produces fever, neuropeptide release, endothelial cell activation, increased adhesion molecule expression, neutrophil priming, hypotension, myocardial suppression, and a catabolic state.8 The term interleukin was coined for a group of cytokines that promotes communication between and among (“inter”) leukocytes (“leukin”). Interleukins are a group of different regulatory proteins that act to control many aspects of the immune and inflammatory responses. The interleukins are polypeptides synthesized in response to cellular activation; they produce their inflammatory effects by activating specific receptors on inflammatory cells and vasculature. T-cell lymphocytes influence the activity of other immunologic and nonimmunologic cells by producing an array of interleukins that they secrete. Different interleukins of this class have been isolated and characterized; they function as short-range or intracellular soluble mediators of the immune and inflammatory responses. The interleukin family of cytokines has been rapidly growing in number because of advances in gene cloning.
cell activators that are synthesized by macrophages to act as secondary messengers and activate endothelial cells and white cells.8 Interleukin-1 and tumor necrosis factor are examples of cytokines considered to be important mediators of the biologic responses to infection and other inflammatory reactions. Liberation of interleukin-1 and tumor necrosis factor produces fever, neuropeptide release, endothelial cell activation, increased adhesion molecule expression, neutrophil priming, hypotension, myocardial suppression, and a catabolic state.8 The term interleukin was coined for a group of cytokines that promotes communication between and among (“inter”) leukocytes (“leukin”). Interleukins are a group of different regulatory proteins that act to control many aspects of the immune and inflammatory responses. The interleukins are polypeptides synthesized in response to cellular activation; they produce their inflammatory effects by activating specific receptors on inflammatory cells and vasculature. T-cell lymphocytes influence the activity of other immunologic and nonimmunologic cells by producing an array of interleukins that they secrete. Different interleukins of this class have been isolated and characterized; they function as short-range or intracellular soluble mediators of the immune and inflammatory responses. The interleukin family of cytokines has been rapidly growing in number because of advances in gene cloning.
Complement
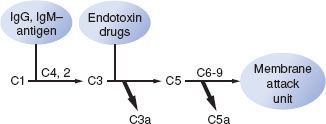
The primary humoral response to antigen and antibody binding is activation of the complement system.9,10 The complement system consists of around 20 different proteins that bind to activated antibodies, other complement proteins, and cell membranes. The complement system is an important effector system of inflammation. Complement activation can be initiated by IgG or IgM binding to antigen, by plasmin through the classic pathway, by endotoxin, or by drugs through the alternate (properdin) pathway (Fig. 12-2). Specific fragments released during complement activation include C3a, C4a, and C5a, which have important humoral and chemotactic properties (see Non-IgE–mediated Reactions section). The major function of the complement system is to recognize bacteria both directly and indirectly by attracting phagocytes (chemotaxis), as well as the increased adhesion of phagocytes to antigens (opsonization), and cell lysis by activation of the complete cascade.9,10
FIGURE 12-2. Diagram of complement activation. Complement system can be activated by either the classic pathway (IgG, IgM–antigen interaction) or the alternate pathway (endotoxin, drug interaction). Small peptide fragments of C3 and C5 called anaphylatoxins (C3a, C5a) that are released during activation are potent vasoactive mediators. Formation of the complete complement cascade produces a membrane attack unit that lyses cell walls and membranes. An inhibitor of the complement cascade, the C1 esterase inhibitor, ensures the complement system is turned off most of the time.
A series of inhibitors regulates activation to ensure regulation of the complement system. Hereditary (autosomal dominant) or acquired (associated with lymphoma, lymphosarcoma, chronic lymphatic leukemia, and macroglobulinemia) angioneurotic edema is an example of a deficiency in an inhibitor of the C1 complement system (C1 esterase deficiency). This syndrome is characterized by recurrent increased vascular permeability of specific subcutaneous and serosal tissues (angioedema), which produces laryngeal obstruction and respiratory and cardiovascular abnormalities after tissue trauma and surgery, or even without any obvious precipitating factor.11 One of the important pathologic manifestations of complement activation is acute pulmonary vasoconstriction associated with protamine administration.1
Effects of Anesthesia on Immune Function
Anesthesia and surgery depress nonspecific host resistance mechanisms, including lymphocyte activation and phagocytosis.12  Immune competence during surgery can be affected by direct and hormonal effects of anesthetic drugs, by immunologic effects of other drugs used, by the surgery, by coincident infections, and by transfused blood products. Blood represents a complex of humoral and cellular elements that may alter immunomodulation to various antigens. Although multiple studies demonstrate in vitro changes of immune function, no studies have ever proved their importance.12 Besides, such changes are likely of minor importance compared with the hormonal aspects of stress responses.
Immune competence during surgery can be affected by direct and hormonal effects of anesthetic drugs, by immunologic effects of other drugs used, by the surgery, by coincident infections, and by transfused blood products. Blood represents a complex of humoral and cellular elements that may alter immunomodulation to various antigens. Although multiple studies demonstrate in vitro changes of immune function, no studies have ever proved their importance.12 Besides, such changes are likely of minor importance compared with the hormonal aspects of stress responses.
HYPERSENSITIVITY RESPONSES (ALLERGY)
Gell and Coombs first described a scheme for classifying immune responses to understand specific diseases mediated by immunologic processes. The immune pathway functions as a protective mechanism, but can also react inappropriately to produce a hypersensitivity or allergic response. They defined four basic types of hypersensitivity, types I to IV. It is useful first to review all four mechanisms to understand the different immune reactions that occur in humans.
Type I Reactions
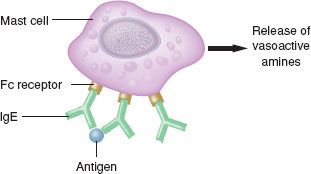
Type I reactions are anaphylactic or immediate-type hypersensitivity reactions (Fig. 12-3). Physiologically active mediators are released from mast cells and basophils after antigen binding to IgE antibodies on the membranes of these cells. Type I hypersensitivity reactions include anaphylaxis, extrinsic asthma, and allergic rhinitis.
FIGURE 12-3. Type I immediate hypersensitivity reactions (anaphylaxis) involve IgE antibodies binding to mast cells or basophils by way of their Fc receptors. On encountering immunospecific antigens, the IgE becomes cross-linked, inducing degranulation, intracellular activation, and release of mediators. This reaction is independent of complement.
Type II Reactions
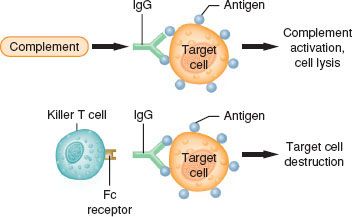
Type II reactions are also known as antibody-dependent cell-mediated cytotoxic hypersensitivity or cytotoxic reactions  (antibody-dependent cell-mediated cytotoxic) (Fig. 12-4). These reactions are mediated by either IgG or IgM antibodies directed against antigens on the surface of foreign cells. These antigens may be either integral cell membrane components (A or B blood group antigens in ABO incompatibility reactions) or haptens that absorb to the surface of a cell, stimulating the production of antihapten antibodies (autoimmune hemolytic anemia). The cell damage in type II reactions is produced by (1) direct cell lysis after complete complement cascade activation, (2) increased phagocytosis by macrophages, or (3) killer T-cell lymphocytes producing antibody-dependent cell-mediated cytotoxic effects. Examples of type II reactions in humans are ABO-incompatible transfusion reactions, drug-induced immune hemolytic anemia, and heparin-induced thrombocytopenia.
(antibody-dependent cell-mediated cytotoxic) (Fig. 12-4). These reactions are mediated by either IgG or IgM antibodies directed against antigens on the surface of foreign cells. These antigens may be either integral cell membrane components (A or B blood group antigens in ABO incompatibility reactions) or haptens that absorb to the surface of a cell, stimulating the production of antihapten antibodies (autoimmune hemolytic anemia). The cell damage in type II reactions is produced by (1) direct cell lysis after complete complement cascade activation, (2) increased phagocytosis by macrophages, or (3) killer T-cell lymphocytes producing antibody-dependent cell-mediated cytotoxic effects. Examples of type II reactions in humans are ABO-incompatible transfusion reactions, drug-induced immune hemolytic anemia, and heparin-induced thrombocytopenia.
FIGURE 12-4. Type II or cytotoxic reactions. Antibody of an IgG or IgM class is directed against antigens on an individual’s own cells (target cells). The antigens may be integral membrane components or foreign molecules that have been absorbed. This may lead to complement activation, including cell lysis (upper figure) or cytotoxic action by killer T-cell lymphocytes (lower figure).
Type III Reactions (Immune Complex Reactions)
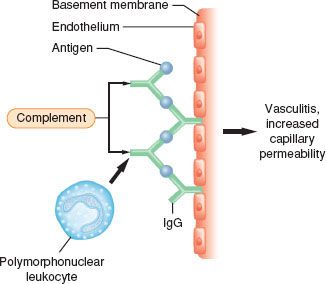
Type III reactions result from circulating soluble antigens and antibodies that bind to form insoluble complexes that deposit in the microvasculature (Fig. 12-5). Complement is activated, and neutrophils are localized to the site of complement deposition to produce tissue damage. Type III reactions include classic serum sickness observed after snake antisera or antithymocyte globulin, and immune complex vascular injury, and may occur through mechanisms of protamine-mediated pulmonary vasoconstriction.1
FIGURE 12-5. Type III immune complex reactions. Antibodies of an IgG or IgM type bind to the antigen in the soluble base and are subsequently deposited in the microvasculature. Complement is activated, resulting in chemotaxis and activation of polymorphonuclear leukocytes at the site of antigen–antibody complexes and subsequent tissue injury.
Type IV Reactions (Delayed Hypersensitivity Reactions)
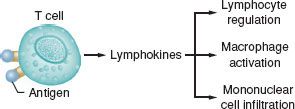
Type IV reactions result from the interactions of sensitized lymphocytes with specific antigens (Fig. 12-6). Delayed hypersensitivity reactions are mainly mononuclear, manifest in 18 to 24 hours, peak at 40 to 80 hours, and disappear in 72 to 96 hours. Antigen–lymphocyte binding produces lymphokine synthesis, lymphocyte proliferation, and generation of cytotoxic T cells, and attracting macrophages and other inflammatory cells. Cytotoxic T cells are produced specifically to kill target cells that bear antigens identical with those that triggered the reaction. This form of immunity is important in tissue rejection, graft-versus-host reactions, contact dermatitis (e.g., poison ivy), and tuberculin immunity.
FIGURE 12-6. Type IV immune complex reactions (delayed hypersensitivity or cell-mediated immunity). Antigen binds to sensitized T-cell lymphocytes to release lymphokines after a second contact with the same antigen. This reaction is independent of circulating antibody or complement activation. Lymphokines induce inflammatory reactions and activate, as well as attract, macrophages and other mononuclear cells to produce delayed tissue injury.
Intraoperative Allergic Reactions
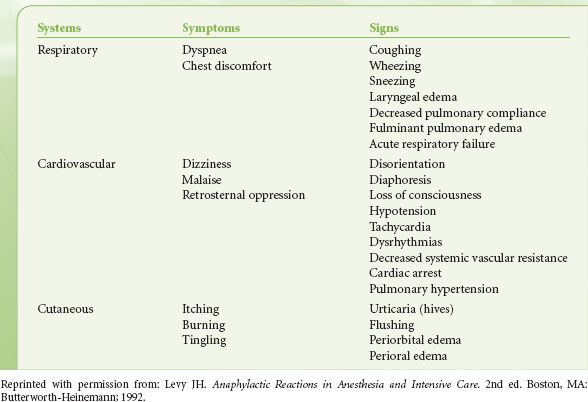
Understanding perioperative anaphylaxis is important because of the potential for morbidity and mortality.13 However, most estimates of the incidence are based on retrospective data, which may account for variability in the incidence. The risk of perioperative anaphylaxis is reported as between 1:3,500 and 1:20,000, with a mortality rate of 4% and an additional 2% surviving with severe brain damage.13,14  More than 90% of the allergic reactions evoked by intravenous drugs occur within 5 minutes of administration. In the anesthetized patient, the most common life-threatening manifestation of an allergic reaction is circulatory collapse, reflecting vasodilation with resulting decreased venous return (Table 12-3). The only manifestation of an allergic reaction may be refractory hypotension. Portier and Richet first used the word anaphylaxis (from ana, “against,” and prophylaxis, “protection”) to describe the profound shock and resulting death that sometimes occurred in dogs immediately after a second challenge with a foreign antigen.15 When life-threatening allergic reactions mediated by antibodies occur, they are defined as anaphylactic. Although the term anaphylactoid has been used in the past to describe nonimmunologic reactions, this term is now rarely used.
More than 90% of the allergic reactions evoked by intravenous drugs occur within 5 minutes of administration. In the anesthetized patient, the most common life-threatening manifestation of an allergic reaction is circulatory collapse, reflecting vasodilation with resulting decreased venous return (Table 12-3). The only manifestation of an allergic reaction may be refractory hypotension. Portier and Richet first used the word anaphylaxis (from ana, “against,” and prophylaxis, “protection”) to describe the profound shock and resulting death that sometimes occurred in dogs immediately after a second challenge with a foreign antigen.15 When life-threatening allergic reactions mediated by antibodies occur, they are defined as anaphylactic. Although the term anaphylactoid has been used in the past to describe nonimmunologic reactions, this term is now rarely used.
TABLE 12-3. RECOGNITION OF ANAPHYLAXIS DURING REGIONAL AND GENERAL ANESTHESIA
ANAPHYLACTIC REACTIONS
IgE-mediated Pathophysiology
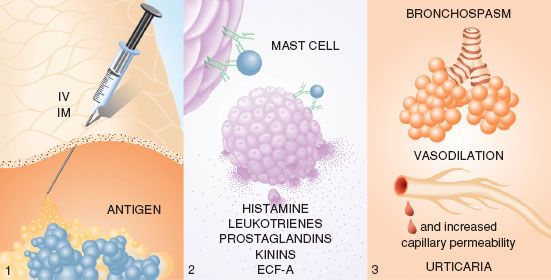
 Antigen binding to IgE antibodies initiates anaphylaxis (Fig. 12-7). Prior exposure to the antigen or to a substance of similar structure is needed to produce sensitization, although an allergic history may be unknown to the patient. On reexposure, binding of the antigen to bridge two immunospecific IgE antibodies found on the surfaces of mast cells and basophils releases stored mediators, including histamine, tryptase, and chemotactic factors.13,16,17 Arachidonic acid metabolites (leukotrienes and prostaglandins), kinins, and cytokines are subsequently synthesized and released in response to cellular activation.18 The released mediators produce a symptom complex of bronchospasm and upper airway edema in the respiratory system, vasodilation and increased capillary permeability in the cardiovascular system, and urticaria in the cutaneous system. Different mediators are released from mast cells and basophils after activation.
Antigen binding to IgE antibodies initiates anaphylaxis (Fig. 12-7). Prior exposure to the antigen or to a substance of similar structure is needed to produce sensitization, although an allergic history may be unknown to the patient. On reexposure, binding of the antigen to bridge two immunospecific IgE antibodies found on the surfaces of mast cells and basophils releases stored mediators, including histamine, tryptase, and chemotactic factors.13,16,17 Arachidonic acid metabolites (leukotrienes and prostaglandins), kinins, and cytokines are subsequently synthesized and released in response to cellular activation.18 The released mediators produce a symptom complex of bronchospasm and upper airway edema in the respiratory system, vasodilation and increased capillary permeability in the cardiovascular system, and urticaria in the cutaneous system. Different mediators are released from mast cells and basophils after activation.
FIGURE 12-7. During anaphylaxis (type I immediate hypersensitivity reaction), (1) antigen enters a patient during anesthesia through a parenteral route. (2) It bridges two IgE antibodies on the surface of mast cells or basophils. In a calcium-dependent and energy-dependent process, cells release various substances— histamine, eosinophilic chemotactic factor of anaphylaxis, leukotrienes, prostaglandins, and kinins. (3) These released mediators produce the characteristic effects in the pulmonary, cardiovascular, and cutaneous systems. The most severe and life-threatening effects of the vasoactive mediators occur in the respiratory and cardiovascular systems. (Reprinted with permission from: Levy JH. Identification and Treatment of Anaphylaxis: Mechanisms of Action and Strategies for Treatment Under General Anesthesia. Chicago, IL: Smith Laboratories; 1983.)
Chemical Mediators of Anaphylaxis
Histamine stimulates H1, H2, and H3 receptors. H1 receptor activation releases endothelium-derived relaxing factor (nitric oxide) from vascular endothelium, increases capillary permeability, and contracts airway and vascular smooth muscle.16,19 H2 receptor activation causes gastric secretion, inhibits mast cell activation, and contributes to vasodilation. When injected into skin, histamine produces the classic wheal (increased capillary permeability producing tissue edema) and flare (cutaneous vasodilation) response in humans.20,21 Histamine undergoes rapid metabolism in humans by the enzymes histamine N-methyltransferase and diamine oxidase found in endothelial cells.
Peptide Mediators of Anaphylaxis
Factors are released from mast cells and basophils that cause granulocyte migration (chemotaxis) and collection at the site of the inflammatory stimulus.17,18 Eosinophilic chemotactic factor of anaphylaxis (ECF-A) is a small molecular weight peptide chemotactic for eosinophils.22 Although the exact role of ECF-A or the eosinophil in acute allergic response is unclear, eosinophils release enzymes that can inactivate histamine and leukotrienes.18 In addition, a neutrophilic chemotactic factor is released that causes chemotaxis and activation.18,23 Neutrophil activation may be responsible for recurrent manifestations of anaphylaxis.
Arachidonic Acid Metabolites
Leukotrienes and prostaglandins are both synthesized after mast cell activation from arachidonic acid metabolism of phospholipid cell membranes through either lipoxygenase or cyclooxygenase pathways.22 The classic slow-reacting substance of anaphylaxis is a combination of leukotrienes C4, D4, and E4. Leukotrienes produce bronchoconstriction (more intense than that produced by histamine), increased capillary permeability, vasodilation, coronary vasoconstriction, and myocardial depression.24 Prostaglandins are potent mast cell mediators that produce vasodilation, bronchospasm, pulmonary hypertension, and increased capillary permeability. Prostaglandin D2, the major metabolite of mast cells, produces bronchospasm and vasodilation. Elevated plasma levels of thromboxane B2 (the metabolite of thromboxane A2), also a prostaglandin synthesized by mast cells as well as by PMNs, have been demonstrated after protamine reactions associated with pulmonary hypertension.
Kinins
Small peptides called kinins are synthesized in mast cells and basophils to produce vasodilation, increased capillary permeability, and bronchoconstriction. Kinins can stimulate vascular endothelium to release vasoactive factors, including prostacyclin, and endothelial-derived relaxing factors such as nitric oxide.
Platelet-activating Factor
Platelet-activating factor (PAF), an unstored lipid synthesized in activated human mast cells, is a potent biologic material. PAF aggregates and activates human platelets, and perhaps leukocytes, to release inflammatory products. PAF levels were significantly higher in patients with anaphylaxis than controls and were correlated with the severity of anaphylaxis.23
Recognition of Anaphylaxis
The onset and severity of the reaction relate to the mediator’s specific end-organ effects. Antigenic challenge in a sensitized individual usually produces immediate clinical manifestations of anaphylaxis, but the onset may be delayed 2 to 20 minutes.24–27 The reaction may include some or all the symptoms and signs listed in Table 12-3. Individuals vary in their manifestations and course of anaphylaxis. A spectrum of reactions exists, ranging from minor clinical changes to the full-blown syndrome leading to death.24,28 The enigma of anaphylaxis lies in the unpredictability of happening, the severity of the attack, and the lack of a prior allergic history.
Non-IgE–mediated Reactions
Other immunologic and nonimmunologic mechanisms release many of the mediators previously discussed independent of IgE, creating a clinical syndrome identical with anaphylaxis. Specific pathways important in producing the same clinical manifestations are considered later.
Complement Activation
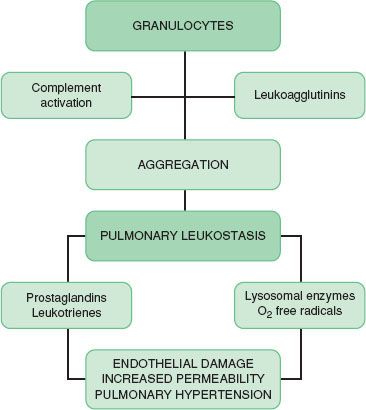
Complement activation follows both immunologic (antibody mediated; i.e., classic pathway) or nonimmunologic (alternative) pathways to include a series of multimolecular, self-assembling proteins that release biologically active complement fragments of C3 and C5.9,10 C3a and C5a are called anaphylatoxins because they release histamine from mast cells and basophils, contract smooth muscle, increase capillary permeability, and cause interleukin synthesis (Table 12-4). C5a interacts with specific high-affinity receptors on PMNs and platelets, causing leukocyte chemotaxis, aggregation, and activation.9,10 Aggregated leukocytes embolize to various organs, producing microvascular occlusion and liberation of inflammatory products such as arachidonic acid metabolites, oxygen free radicals, and lysosomal enzymes (Fig. 12-8). Antibodies of the IgG class directed against antigenic determinants or granulocyte surfaces can also produce leukocyte aggregation.29 These antibodies are called leukoagglutinins. Investigators have associated complement activation and PMN aggregation in producing the clinical expression of transfusion reactions, pulmonary vasoconstriction after protamine reactions, adult respiratory distress syndrome, and septic shock.1
FIGURE 12-8. Sequence of events producing granulocyte aggregation, pulmonary leukostasis, and cardiopulmonary dysfunction. (Reprinted from: Levy JH. Anaphylactic Reactions in Anesthesia and Intensive Care. 2nd ed. Boston, MA: Butterworth-Heinemann; 1992.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






