Fig. 7.1
Summary of the most important pathophysiologic effects of increased intra-abdominal pressure on end-organ function within and outside the abdominal cavity. Figure legend: Cdyn Dynamic respiratory compliance, CO Cardiac output, CPP Cerebral perfusion pressure, CVP Central venous pressure, GFR Glomerular filtration rate, HR Heart rate, ICP Intracranial pressure, ITP Intrathoracic pressure, MAP Mean arterial pressure, PIP Peak inspiratory pressure, Paw Airway pressures, PCWP Pulmonary capillary wedge pressure, pHi Intramucosal gastric pH, Qs/Qt Shunt fraction, RVR Renal vascular resistance, SMA Superior mesenteric artery, SVR Systemic vascular resistance, Vd/Vt Dead space ventilation
Table 7.1
Factors that affect chest wall compliance
• Pleural effusion |
• Lung transplant |
• Sternotomy (post-CABG) |
• Obesity |
• Ascites |
• Fluid overload |
• Rib fractures |
• Abdominal distension |
• Intra-abdominal hypertension (IAH) |
• Abdominal compartment syndrome (ACS) |
Table 7.2
Pulmonary effects of intra-abdominal hypertension and abdominal compartment syndrome. Adapted from Pelosi et al. [1]
Pulmonary effects related to increased IAP |
|---|
Diaphragm elevation ↑ |
Intrathoracic pressure ↑ |
Pleural pressure ↑ |
Peak airway pressure ↑ (volume-controlled MV) |
Mean airway pressure ↑ |
Plateau airway pressure ↑ |
Functional residual capacity (FRC) ↓ |
All lung volumes (TLC, TV, etc.) ↓ (~restrictive disease) |
Extrinsic compression lung parenchyma ↑ |
Auto-PEEP ↑ |
Compression atelectasis ↑ |
Pulmonary vascular resistance ↑ |
Alveolar baro−/volutrauma = ↑ |
Compliance ↓ |
Respiratory system compliance ↓ |
Chest wall compliance ↓↓ |
Lung compliance = |
Upper inflection point on PV curve ↓ |
Lower inflection point on PV curve ↑ |
Hypercarbia—pCO2 retention ↑ |
PaO2 ↓ and PaO2/FiO2 ↓ |
Alveolar oxygen tension ↓ |
Oxygen transport ↓ |
Dead space ventilation ↑ |
Intrapulmonary shunt ↑ |
Ventilation perfusion mismatch ↑ |
Ventilation diffusion mismatch ↑↑ |
Oxygen consumption ↑ |
Metabolic cost and work of breathing ↑ |
Alveolar oedema ↑ |
Extravascular lung water (EVLW) = ↗ |
Pulmonary vascular permeability index (PVPI) = ↗ |
Prolonged ventilation |
Difficult weaning |
Activated lung neutrophils (experimental) ↑ |
Pulmonary inflammatory infiltration (experimental) ↑ |
Pulmonary infection rate (experimental) ↑ |
Table 7.3
Cardiovascular effects of intra-abdominal hypertension and abdominal compartment syndrome. Adapted from Malbrain et al. [2]
Cardiovascular effects related to increased IAPa |
|---|
Diaphragm elevation and cardiac compression ↑ |
Pleural and intrathoracic pressure (ITP) ↑ |
Difficult preload assessment |
Pulmonary capillary wedge pressure (PCWP) ↑ |
Central venous pressure (CVP) ↑ |
Mean systemic filling pressure ↑ |
Transmural filling pressure = ↘ |
Intrathoracic blood volume (ITBV) = ↘ |
Global end-diastolic volume (GEDV) = ↘ |
Right ventricular end-diastolic volume (RVEDV) = ↘ |
Right, global and left ventricular ejection fraction = ↘ |
Extravascular lung water (EVLW) = ↗ |
Stroke volume variation (SVV) ↗ |
Pulse pressure variation (PPV) ↗ |
Systolic pressure variation (SPV) ↗ (Δdown =, Δup ↑) |
Inferior vena caval flow ↓ |
Venous return ↓ |
Left ventricular compliance and contractility ↓ |
Downward and rightward shift of Frank-Starling curve |
Cardiac output ↓ |
Systemic vascular resistance (SVR) ↑ |
Mean arterial pressure (MAP) ↗ = ↘ |
Pulmonary artery pressure (PAP) ↑ |
Pulmonary vascular resistance (PVR) ↑ |
Heart rate ↗ = |
Lower extremity hydrostatic venous pressure ↑ |
Venous stasis, oedema, ulcers ↑ |
Venous thrombosis ↑ |
Pulmonary embolismb ↑ |
Mixed venous oxygen saturation ↓ |
Central venous oxygen saturation ↓ |
False negative passive leg raising test ↑ |
Functional haemodynamic thresholds for fluid responsiveness ↑ |
7.2 Epidemiology
Around one in four patients will have signs and symptoms of IAH on ICU admission, whilst around one out of two will develop IAH within the first week of ICU stay [3]. Moreover, 1 in 20 patients will develop overt ACS, a lethal syndrome with a mortality rate above 75% when left untreated [4]. To this day, patients may have unrecognised IAH. The major risk factors of IAH include abdominal surgery, surgery performed in the emergency setting, severe poly-trauma, abdominal trauma, severe haemorrhagic shock, severe burns, severe acute pancreatitis, large volume fluid resuscitation (especially crystalloid) resulting in fluid overload, ileus and liver dysfunction [5].
7.3 Consensus Definitions
Recently the World Society of the Abdominal Compartment Syndrome (WSACS) changed its name into the Abdominal Compartment Society (www.wsacs.org) [6]. As the focus concerning ACS becomes less paramount as it becomes less frequent, it became even more apparent that the actual name of the Society was limiting in terms of reflecting the true breadth and depth of the Society’s mission. The ACS emphasises the most dramatic condition to be addressed, but it does not reflect upon the full scope of the Society’s interests and activities [6]. In order to reflect the evolving science and to embrace important concepts related to abdominal wall anatomy and function, the focus was broadened from ACS to formally appreciating the abdominal compartment as a whole within all the body’s interrelated compartments [6]. Hereby follows a short list of the latest consensus definitions as formulated by the WSACS, the Abdominal Compartment Society [7].
Definition 1: Intra-abdominal pressure
IAP is the steady-state pressure concealed within the abdominal cavity.
Definition 2: Abdominal perfusion pressure
In analogy to cerebral perfusion pressure, abdominal perfusion pressure (APP) is defined as mean arterial pressure (MAP) minus IAP.
Definition 3: IAP measurement
IAP should be expressed in mmHg and measured at end expiration in the complete supine position after ensuring that abdominal muscle contractions are absent and with the transducer zeroed at the level where the mid-axillary line crosses the iliac crest.
Definition 4: Gold standard IAP measurement method
The reference standard for intermittent IAP measurements is via the bladder with a maximal instillation volume of 25 mL of sterile saline.
Paediatric-specific definition: The reference standard for intermittent IAP measurement in children is via the bladder using 1 mL/kg as an instillation volume, with a minimal instillation volume of 3 mL and a maximum installation volume of 25 mL of sterile saline.
Definition 5: Normal IAP
Normal IAP is approximately 5–7 mmHg and around 10 mmHg in critically ill adults.
Paediatric-specific definition: IAP in critically ill children is approximately 4–10 mmHg.
Definition 6: Intra-abdominal hypertension
IAH is defined by a sustained or repeated pathologic elevation of IAP ≥12 mmHg.
Paediatric-specific definition: IAH in children is defined by a sustained or repeated pathological elevation in IAP >10 mmHg.
Definition 7: IAH grading
IAH is graded as follows:
Grade I: IAP 12–15 mmHg
Grade II: IAP 16–20 mmHg
Grade III: IAP 21–25 mmHg
Grade IV: IAP >25 mmHg
Definition 8: Abdominal compartment syndrome
ACS is defined as a sustained increased IAP ≥20 mmHg (with or without an APP <60 mmHg) that is associated with new organ dysfunction or failure.
Paediatric-specific definition: ACS in children is defined as a sustained elevation in IAP of greater than 10 mmHg associated with new or worsening organ dysfunction that can be attributed to elevated IAP.
Definition 9: Primary IAH/ACS
Primary IAH/ACS (formerly also known as surgical or abdominal) is a condition associated with injury or disease in the abdomino-pelvic region that frequently requires early surgical or interventional radiological intervention.
Definition 10: Secondary IAH/ACS
Secondary IAH/ACS (formerly also known as medical or extra-abdominal) refers to conditions that do not originate from the abdomino-pelvic region.
Definition 11: Recurrent IAH/ACS
Recurrent IAH/ACS (formerly also known as tertiary) refers to the condition in which IAH/ACS redevelops following previous surgical or medical treatment of primary or secondary IAH/ACS.
Definition 12: Polycompartment syndrome
A polycompartment syndrome is a condition where two or more anatomical compartments have elevated compartmental pressures. This will be discussed further.
Definition 13: Abdominal compliance
Abdominal compliance (Cab) quantifies the ease of abdominal expansion and is determined by the elasticity of the abdominal wall and diaphragm. Cab is expressed as the change in intra-abdominal volume per change in intra-abdominal pressure in L/mmHg.
Definition 14: Open abdomen
An open abdomen (OA) is any abdomen requiring a temporary abdominal closure (TAC) due to the skin and fascia not being closed after laparotomy. The technique of temporary abdominal closure should be explicitly described.
Definition 15: Open abdomen classification
The open abdomen is classified with the following grading system:
1—No fixation.
1A: Clean, no fixation.
1B: Contaminated, no fixation.
1C: Enteric leak, no fixation.
2—Developing fixation.
2A: Clean, developing fixation.
2B: Contaminated, developing fixation.
2C: Entero-atmospheric/cutaneous fistula, developing fixation.
3 and 4—Frozen abdomen.
3: Frozen abdomen, no fistula.
4: Frozen abdomen with entero-atmospheric/cutaneous fistula.
Definition 16: Lateralization
Lateralization of the abdominal wall refers to the phenomenon whereby the musculature and fascia of the abdominal wall, most well seen by the rectus abdominis muscles and their enveloping fascia, move laterally away from the midline with time.
7.4 Effects of Intra-abdominal Hypertension on Respiratory Mechanics
7.4.1 Intra-abdominal Hypertension and Ventilator-Induced Lung Injury (VILI)
Animal studies have shown that increasing IAP during mechanical ventilation may result in cytokine release and subsequent lung injury. Rezende-Neto et al. showed in a study of 50 rats that 60–90 min of IAH (IAP of 20 mmHg via insufflated intraperitoneal air) resulted in increased plasma levels of IL-6, increased polymorphonuclear leucocyte activity in the lungs as evaluated by myeloperoxidase (MPO) assay [8].and intense pulmonary inflammatory infiltration including atelectasis and alveolar oedema on lung histology. Schachtrupp et al. showed in a study of 12 pigs that 24 hours of IAH (IAP of 30 mmHg) also resulted in histological findings similar to those found in VILI (interstitial and alveolar leucocytes and fibrin) but also proximal tubular necrosis in the kidneys and paracentral necrosis in the liver [9]. Since the strain on lung structures leading to VILI depends on transpulmonary pressure, it is not unreasonable to imagine that the frequently used relative too low transpulmonary pressures in the context of IAH will cause shear stress with increased repetitive opening and closing of alveoli units.
7.4.2 Effect of Intra-abdominal Hypertension on Respiratory Mechanics
As stated above lung distension is in part regulated by chest wall mechanics. The stiffer the chest wall, the less lung distension will occur during mechanical ventilation for a given airway pressure. In a Chinese study of 16 patients undergoing decompressive laparotomy (DL), different lung volumes were calculated with computed tomography (CT) at baseline, before and after DL. Compared to controls (n = 6), patients (n = 16) had lower total lung and higher non-aerated lung volumes [10]. This is illustrated in Fig. 7.2. Whereas chest wall elastance accounts in normal conditions for only 15% of the total respiratory system elastance, this number may increase up to 50% during IAH with IAP above 20 mmHg (due to the stiffening of the chest wall). According to the polycompartment model (as will be discussed further down), IAH can increase intrathoracic pressure (ITP) and subsequently increase alveolar pressures [11]. We previously showed in a pig study (n = 11) that IAH up to 30 mmHg (with abdominal saline infusion intraperitoneally) resulted in an abdomino-thoracic transmission index (ATI) between 17% and 62% when looking at end-expiratory vs end-inspiratory oesophageal pressures, respectively [12]. With increasing IAP, both total respiratory system (CRS) and chest wall (CCW) compliance decreased significantly. The decrease was more pronounced for the chest wall and showed a strong inverse correlation with IAP (r = −0.84, p < 0.0001). A pilot study in 14 mechanically ventilated patients with acute lung injury (ALI) showed that the application of an abdominal Velcro belt increased IAP from 8.6 to 15.4 mmHg with a concomitant increase in alveolar plateau pressures (Pplat) from 18 to 23.3 cmH2O (data on file). These changes were paralleled by a decrease in dynamic respiratory compliance from 37 to 28 mL/cmH2O. This probably explains why the suggested lung-protective ventilation strategies are difficult to apply in patients with IAH or those with diminished chest wall compliance like in morbid obesity. Previous animal and human ‘PV curve’ studies focusing on the importance of IAH showed that abdominal and subsequently chest wall compliance improves after abdominal decompression [13, 14].
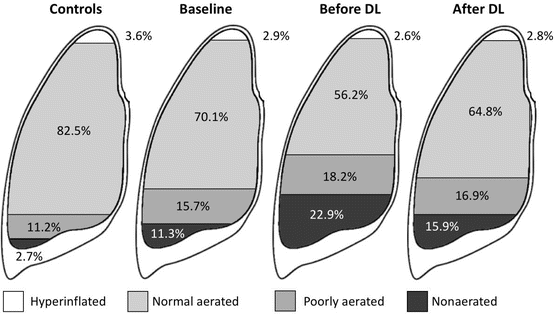

Fig. 7.2
Effect of abdominal hypertension and decompressive laparotomy on total lung volumes expressed percentages of different aerated lung volumes. Adapted from Zhou et al. [10]
Acute respiratory distress syndrome (ARDS) is a syndrome and not a disease, and as a consequence, not all ARDS patients are the same which may be a possible explanation why there are conflicting results in previous ARDS studies. Ranieri et al. found that patients with ARDS had different respiratory mechanics depending upon the underlying aetiology and the presence of IAH. He found that surgical patients had stiffer chest walls compared to medical patients, probably due to the increased presence of abdominal distension [13]. Respiratory system and chest wall compliance improved after DL in these patients. Unfortunately, the effect of positive end-expiratory pressure (PEEP), forced residual capacity (FRC) and IAP was not measured. Mergoni and colleagues studied respiratory system mechanics partitioned between the lung and chest wall and showed that in a subgroup of ARDS patients in which the lower inflection point (LIP) was mainly determined by chest wall (CCW) PEEP was not as effective in improving pO2 [15]. In contrast, in ARDS patients in which LIP was determined by the lung compliance (CL), PEEP was effective.
These findings are somewhat in contradiction with those found by Gattinoni and co-workers, and this can in part be explained by the difference in measurement manoeuvres and techniques as well as the assumptions used [16]. Gattinoni showed that the localised character of parenchymal involvement in primary ARDS (with primary lung involvement, e.g. pneumonia) resulted in decreased lung but normal chest wall compliance, whilst secondary ARDS (mainly as a result of abdominal sepsis) presented with preserved lung but decreased chest wall compliance, and PEEP allows to recruit lung units markedly only in secondary but not in primary ARDS [16]. The results imply that the application of PEEP in pulmonary ARDS may cause over-distension of already open lung units, making these patients more prone to ventilator-induced lung injury (VILI) than patients with extrapulmonary ARDS and IAH. The same phenomenon may be responsible for the change in respiratory mechanics seen in morbidly obese patients [17]. Measuring IAP may therefore provide an easy bedside method to estimate altered chest wall mechanics and avert the need to measure oesophageal pressure (as surrogate for ITP). Measuring oesophageal pressure is not easy due to some practical problems at the bedside [1]. IAP also influences the shape of the PV curve (with downward flattening and rightward shifting) of the total respiratory system and the chest wall, whilst the lung mechanics basically remain unaffected [18].
7.4.3 Effect of Intra-abdominal Hypertension on Lung Recruitment
The most frequent performed recruitment manoeuvre is a 40-by-40 manoeuvre (40 s holding 40 cmH2O inspiratory pressure). It is estimated that transpulmonary opening pressure equal to 30 cmH2O is required to open atelectasis. In the setting of IAH with altered CL/CRS ratio from 0.85 to 0.5, the resulting transpulmonary pressure during a 40-by-40 recruitment manoeuvre may only be 20 cmH2O; hence, the alveolar units with long-time constants would remain collapsed [19]. Therefore in the setting of IAH, higher opening pressures closer to 40 cmH2O + IAP/2 may be required [20]. Lung-protective ventilation implies opening the lungs (with a recruitment manoeuvre or thus peak alveolar pressures) and keeping the lungs open (with appropriate PEEP setting) [21]. The altered lung mechanics and the different recruitment manoeuvres needed in IAH have also an impact on lung-protective ventilation (limiting Pplat below 30 cmH2O) as this will result in very low tidal volumes (TV) in the setting of IAH or ACS. Therefore, Pplat should be limited towards a maximal peak alveolar pressure of ‘30 cmH2O + IAP/2’, or stated otherwise, transpulmonary Pplat calculated as Pplat—IAP/2 should be kept below 30 (to 35) cmH2O. This statement is supported by the fact that Talmor and co-workers found that IAP (e.g. measured via the stomach) and oesophageal pressure (e.g. measured via an oesophageal balloon) are closely correlated [22]. Therefore, not only opening pressures but also closing pressures are increased during IAH and ACS and as such higher PEEP levels are required to prevent end-expiratory lung collapse. Keeping the lungs open is equally important after a recruitment manoeuvre to avoid shear stress of opening and closing lung units that may induce VILI. As a rule of thumb, PEEP (in cmH2O) can be set equal to IAP (in mmHg). This assumption takes into account the fact that the ATI is not 100% as the conversion factor from mmHg to cmH2O is 1.36. Some experimental data suggested the use of higher TVs around 10 mL/kg (as compared to 6 mL/kg) in IAH/ACS, but this strategy cannot be recommended yet in patients [23].
7.4.4 Effect of Intra-abdominal Hypertension on Lung Oedema and Lymphatic Drainage
A landmark paper by Quintel and co-workers showed that IAH causes an increase in lung oedema in a pig model of acute lung injury (induced by oleic acid) [18]. When IAP was increased from 0 to 20 cmH2O, lung oedema distribution changed from the dorsobasal regions to the complete lung. In keeping with this, Schachtrupp showed an increase in extravascular lung water (EVLW) in and histological lung alterations at IAP levels of 30 cmH2O [24, 25]. An epidemiologic study in humans also found a correlation between IAP, fluid balance and EVLW in patients with acute lung injury, suggesting a link between sepsis, capillary leak, fluid overload, abdominal hypertension and lung oedema [26]. This may explain why active fluid removal or so-called de-resuscitation with PAL treatment (PEEP in cmH2O set at the level of IAP in mmHg, followed by hyperoncotic albumin 20% and Lasix®) was able to reduce IAP and EVLWI in a pilot study of 57 patients matched with historical controls [27, 28].
Fluid drainage from the lungs can take place via three mechanisms: transpleural, via the lung hilum or transabdominal [29]. The effects of different ventilatory settings and increasing IAP on thoracic and abdominal lymph flow were studied in a porcine endotoxin sepsis model [30]. The study was performed in three parts and data were collected from a total of 32 pigs. In summary the authors found that lipopolysaccharide infusion increased IAP and lymphatic flow, that PEEP increased IAP and lymph production but impeded lymphatic drainage across the diaphragm, that spontaneous breathing improved transdiaphragmatic lymph drainage and finally that increased IAP diminished lymphatic flow. Although often overlooked, the role of lymphatic flow is complex but very important to determine not only the fluid balance in the lung but also in the peripheral organs [31]. Different pathologies and treatments can markedly influence the pathophysiology of the lymphatics with dramatic effects on end-organ function.
7.5 Effects of Intra-abdominal Hypertension on Cardiovascular Dynamics
7.5.1 Effect of Intra-abdominal Hypertension on Cardiac Contractility
Diaphragmatic elevation and increased ITP exert direct mechanic effects on cardiac contractility. This will be accompanied by an increase in pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR) with a simultaneous decrease in left ventricular preload, finally leading to a decrease in cardiac output (CO). Animal studies have demonstrated a rightward and downward shift of the Frank-Starling curve in a dog model of IAH up to 40 mmHg created with fluid infusion in the peritoneal cavity [32]. Left ventricle wall motion assessment with trans-oesophageal echocardiography showed a significant decrease at the level of the anteroseptal wall in eight children during laparoscopic hernioraphy (with IAP levels of only 12 mmHg) [33]. Increases in central venous pressure (CVP) and IAP have also been observed in patients with congestive heart failure developing acute renal failure [34]. Whilst initially responsive to fluid loading and inotropic (dobutamine but not dopamine) support at lower levels of IAH, the deleterious cardiovascular effects in patients with ACS can only be effectively treated by nonoperative measures to reduce IAP or abdominal decompression. The APP seems a promising target to guide resuscitation in combination with less invasive haemodynamic monitoring like the transpulmonary thermodilution technique. After induction of IAH via pneumoperitoneum in ten pigs, an increase in CO following fluid loading was only indicated by calibrated CO but not by uncalibrated continuous CO methods using arterial waveform analysis [35].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






