Fig. 8.1
Severity of OSA based on AHI
It is well known that OSA is often associated with severe complications, major cardiovascular disorders (there is a strong correlation between the disease and hypertension, coronary artery disease, heart failure, arrhythmias, and stroke), neurocognitive impairments (attention and concentration changes, executive function and fine motor coordination), and mood disorders as depression [4].
The amount of patients diagnosed with OSA has increased drastically and is becoming a public health issue with potential social consequences [2]. The recognition of this syndrome is essential in patients undergoing elective surgery in whom prevalence of sleep apnea is much higher. Indeed, sedation and anesthesia have been shown to increase the upper airway collapsibility and therefore increase the risk of having postoperative complications in these patients. It is important to identify these patients preoperatively so that appropriate actions can be taken [3, 5, 6].
8.2 Epidemiology
The prevalence of OSA has increased in epidemiological studies over time. Differences and the increase in prevalence of sleep apnea are probably due to different diagnostic equipment, definitions, study design, and characteristics of included subjects including effects of the obesity epidemic [2]. The estimated prevalence of OSA syndrome (AHI ≥5 and excessive daytime sleepiness) has been reported between 6 and 22% in men and 4 and 17% in women. This disease is often asymptomatic, and the prevalence of patients with OSA, who do not present clinical syndrome, has increased in the last studies. It was reported in 37% of men and in 50% of women in the middle-aged population [1, 2, 7].
8.2.1 Risk Factors Associated with OSA
Surgical patients are at higher risk of having complications for a variety of reasons, which are essential comorbidities, especially chronic obstructive pulmonary disease, coronary artery disease, and renal failure. Considering this, it is extremely important to identify correctly patients preoperatively. Patients with OSA are more frequently male, obese, and 65 years old or more. OSA is also related to race. African-Americans are more frequently affected and develop the disease at a younger age than other races [4].
The most important factors associated with OSA observed in studies over time are related with obesity, gender, age, smoking and alcohol abuse, and cardiovascular disease (Fig. 8.1).
8.2.1.1 Obesity
Obesity is the most important risk factor for snoring and sleep apnea and a majority of patients with OSA are overweight. A body mass index (BMI) of ≥25 kg/m2 is associated with moderate-to-severe OSA in more than 50% cases. Fat distribution, in particular, deposition around the trunk and neck, predisposes to upper airway obstruction and OSA because of mass loading. Neck or waist circumferences are better predictors of OSA breathing as compared with BMI alone. Neck circumference seems to be the most important risk factor for snoring in recent studies between populations [1].
In addition, it has been shown that caloric restriction or bariatric surgery in combination with positive airway pressure (CPAP) therapy obtains better results, reducing the severity of OSA than CPAP alone in overweight people.
Nevertheless, not only subjects with obesity and fat necks suffer from sleep apnea but also lean subject, and about one-third of OSA syndrome patients are nonobese [2].
8.2.1.2 Gender
OSA is more common in men than women with an estimated ratio about 2:1. The prevalence of snoring shows similar gender differences. Possible explanations for the male predominance include hormonal effects in the upper airway, gender differences in body fat distribution, and differences in pharyngeal anatomy and function. Hormonal influences could play an important role in the pathogenesis of OSA; indeed, postmenopausal women are also at higher risk, but the pathophysiological roles of hormones are, however, unclear [2, 7].
In recent studies, it has been reported that sleep apnea occurs in as much as 50% of females aged 20–70 years old in the population. OSA symptom differs between males and females; daytime sleepiness is rare in females; instead, hypertension, obesity, and age were associated with sleep apnea in females. Moreover an epidemiologic study reported that 39% of normal-weighted women had OSA, but only 0.1% of them had severe sleep apnea [1, 2, 8].
8.2.1.3 Age
The risk of OSA increases as well with increasing age. Snoring frequency increases with age up to 50–60 years old and then decreases after. Recent studies reported an increase in OSA after 65 years; in contrast, the frequency of OSA syndrome declined. An association between sleep-disordered breathing and morbidity and mortality at older ages has been observed, so that sleep apnea in seniors represents a specific entity compared with middle-aged adults [2].
Obstructive sleep apnea (OSA) is a common pediatric health problem, and children at risk need to be identified, investigated, and treated in a timely manner because the resultant activation of inflammatory cascades can impose wide-ranging effects, impacting the neurocognitive, cardiovascular, and metabolic systems [2]. Although the etiologies of pediatric OSA are multiple, they can be broadly classified into conditions which result in intrinsic upper airway narrowing and those that result in increased upper airway collapsibility. Adenotonsillar hypertrophy is currently the most common example of the former. Other anatomical features resulting in upper airway narrowing such as micrognathia, macroglossia, and midface hypoplasia are often found in children with craniofacial syndromes (e.g., Treacher Collins syndrome, Crouzon syndrome, Apert syndrome, Pierre Robin sequence), achondroplasia, trisomy 21, Beckwith-Wiedemann syndrome, and mucopolysaccharidoses [9].
8.2.1.4 Smoking
Several epidemiological studies observed significant associations between cigarette smoking and snoring or sleep apnea. Moreover there is a dose-response relationship between smoking and the severity of OSA. Heavy smokers have greatest risk to snoring and sleep disorders. The main reasons possible include airway inflammation and sleep instability from overnight nicotine withdrawal. Never-smokers who have been exposed to passive smoking on a daily basis display an increase in the odds of being a habitual snorer after adjusting for age and BMI.
8.2.1.5 Alcohol
Alcohol produces hypotonia of the oropharyngeal muscles as a result of reducing motor output to the upper airway. It also increases both the number of apneas and the duration of them. However, when we study the relationship between chronic alcohol and snoring or OSA, we found an association in some of the patients but not in others, so it is not clear [1, 2].
In other studies, it is observed that alcohol is related to snoring in lean women, in whom there was no compromised upper airway because of fat deposits or overweight.
8.2.1.6 Cardiovascular Disease
Hypertension and OSA are both prevalent and many people suffer from them together. Compared with subjects with no OSA, the odds ratio for the prevalence of hypertension is >2 for both mild and moderate-to-severe OSA. A causal relationship between OSA and hypertension has been indicated in observational studies but however has also observed not get such as effective results in reducing hypertension when we treat OSA with CPAP [2].
Another positive association is founded between coronary artery disease and OSA. They frequently coexist, but OSA is usually being undiagnosed. Patients with OSA had a higher incidence of coronary artery disease (16.2%) compared with snorers without OSA (5.4%) in a prospective studies [1].
Clinical studies suggest an important link between sleep apnea and stroke, which are the only two risk factors that negatively affected mortality. Studies concluded that OSA syndrome significantly increases the risk of stroke or death, and it is independent of other risk factors, including hypertension [4].
Different types of arrhythmias have been described in patients with OSA. Atrial fibrillation and various degrees of heart block seem to be the more common. Many observational studies report an increase in vagal tone during apneic events, being the possible mechanism in the development of bradyarrhythmias [2, 4].
OSA and diabetes mellitus share several risk factors. These factors usually coexist with snoring, and in general population studies, they are observed as independent of obesity and other factors. There is an association reported between snoring and diabetes in both males and females. Studies also concluded that OSA as a risk factor for future diabetes development is not conclusive [2, 4].
8.3 Pathogenesis
OSA is characterized by recurrent periods of upper airway occlusion during sleep. When the pharynx collapses, airflow obstruction elicits neuromuscular responses that can mitigate the obstruction and restore airway patency and ventilation [7]. If these neuromuscular mechanisms are inadequate, additional factors contribute to the development of recurrent periods of airway obstruction and arousals from sleep.
We consider that the upper airway collapses dynamically during sleep and reopens during wakefulness. Investigators have previously modeled dynamic alterations in patency as a function of transmural pressure across collapsible segments in biologic conduits in the cardiovascular, gastrointestinal, and genitourinary systems. The human pharynx is considered as a collapsible tube whose purposes are speech, swallowing, and respiration. There is not provided with a rigid structure as a skeletal support and during inhalation collapse [1, 7].
In the upper airway, the collapsible segment is bordered by two rigid segments: upstream, the nasal passages, and downstream, like the trachea. The segments upstream and downstream to the collapsible site have fixed diameters and resistances, RUS and RDS, respectively, and the pressures upstream and downstream are PUS and PDS, respectively. When PUS and PDS are less than the critical pressure surrounding the collapsible segment (PCRIT), the transmural pressure is negative, the airway closes, and the airflow ceases. Flow can be reestablished by raising PUS above PCRIT. Taking into account this model, flow through the upper airway is proportional to the pressure gradient across the entire airway. Moreover, when PUS is greater than PCRIT and PDS is less than PCRIT, however, the airway enters a flow-limited condition, and flow would cease transiently. As the upper airway occludes, the pressure immediately upstream of the occlusion would equilibrate with PUS and rise above PCRIT. This increase in pressure would inevitably lead to reopening of the airway (Fig. 8.2).
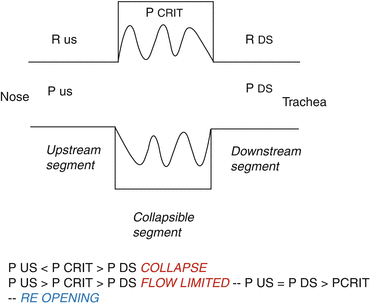

Fig. 8.2
Starling resistor model of the upper airway
Current evidence suggests that disturbances in PCRIT play a primary role in OSA pathogenesis. Elevations in PCRIT have been demonstrated in OSA patients compared to age, sex, and body mass index (BMI)-matched controls under general anesthesia and neuromuscular blockade as well as during sleep. Additional evidence for the primacy of upper airway collapse in OSA pathogenesis is provided by studies demonstrating a dose-response relationship between pharyngeal collapsibility and severity of OSA. As PCRIT rises progressively, increases in severity of upper airway obstruction during sleep have also been observed clinically [3, 7].
In addition, evidence shows that treatments that decrease PCRIT (e.g., weight loss or uvulopalatopharyngoplasty) lead to improvements in OSA and to resolution of disease. Similarly, a positive transmural pressure can be induced by increasing PUS, leading to resolution of upper airway obstruction. With application of progressively increasing nasal pressure during CPAP titration, upper airway obstruction and recurrent obstructive apneas and hypopneas are reversed [3, 7, 10].
Many conditions such as negative pressure, soft tissues, and bony structures predispose the pharynx to collapse. However, the tonic and phasic contractile activity of the dilator muscles produces the opposite effect. When an imbalance between these forces is developed, the upper airway obstruction is produced and recurs in patients with sleep-disordered breathing [1].
In fact, both anatomic and neuromuscular factors are involved and the development of OSA. The findings suggest that elevations in pressure in the collapsible segment of the pharynx of OSA patients are due to defects in both upper airway structural and neuromuscular controls, and both play a pivotal role in OSA pathogenesis. OSA can only develop when neuromuscular responses do not adequately mitigate the obstruction caused [2, 7].
Anatomic alterations have been identified such as a variety of factors that contribute to increased collapsibility. In a narrow upper airway, according to the Venturi effect, while airflow velocity increases, pressure on the lateral wall of the pharynx decreases, and the collapsibility does it as well. Excessive fat deposits, particularly parapharyngeal ones, also contribute to OSA development. In addition, lung volumes are decreased in obese persons, leading to decreased caudal traction on the upper airway and an increased critical closing pressure. Another anatomic factor associated with OSA is the presence of tonsillar and tongue hypertrophy, retrognathia, or inferior displacement of the hyoid bone [2, 7].
An impairment in neuromuscular control responses account for much of the balance of the OSA variability. This kind of patients depends on neuromuscular activity to maintain airway patency and ventilation during sleep. Reductions in neuromuscular tone, especially with reduction in pharyngeal dilator tone at sleep onset, are suspected to contribute to increased OSA severity during REM compared to NREM sleep in selected patients and particularly in women and children. Current studies also suggest that endogenous neurohumoral agents can contribute to modulation of neuromuscular responses. Neurohormonal modulation of pharyngeal neuromuscular activation may take part in prevalence and severity of OSA and differences between men and women. It could be explained because of elevations in circulating leptin levels in women compared to men [1, 7].
Neuromuscular responses are also influenced by pharmacologic modulators of sleep-wake state. Alcohol, sedative medications, and hypnotics could decrease responses to upper airway occlusion and promote upper airway obstruction during sleep. Benzodiazepines are known to prolong obstructive apneas and hypopneas. Opiates have not been well studied in association with upper airway collapsibility. Nevertheless, blockade of opioid receptors has been demonstrated to decrease pressure in the airway, which suggests that it may increase susceptibility to pharyngeal occlusion [5, 7].
Pharyngeal neuromuscular activity is also controlled by chemical and mechanical reflexes. Hypercapnia is also a potent stimulator of upper airway neuromuscular activity.
Hypocapnia, on the other hand, produces a relatively passive state and is associated with elevations in pressure. Pharyngeal sensory inhibition has been demonstrated to decrease neuromuscular responses to upper airway obstruction [7].
8.4 Clinical Manifestations
OSA manifestations develop in a variety of ways from unrecognized symptoms to classic findings. However, the most common presentation is not the recognizable one, and usually OSA progresses over years, delaying diagnosis and producing adverse effects.
As epidemiologic studies are observed, OSA is underdiagnosed, and it is necessary that clinicians are familiar with both the subtle and overt clinical manifestations of OSA to accurately identify patients at risk for the disease and order appropriate testing, to decrease the risk of postoperative mortality in patients with a known diagnosis of sleep apnea [2, 4]. Perioperatively identification of patients is mandatory in order to optimal management [5].
Many classic symptom and exam findings are shown in OSA patients (Table 8.1). The association between OSA and different disorders has been shown in several studies, especially cardiovascular and neurocognitive impairments but also metabolic and endocrine disturbances [4, 11].
Table 8.1
Classic symptoms and exam findings in OSA
Classic symptoms | Exam findings |
|---|---|
Snoring Excessive daytime sleepiness Choking or gasping at night Night sweats Neurocognitive impairments Heartburn Morning headaches Maintenance insomnia Erectile dysfunction Nocturia | Obesity Enlarged neck circumference Crowded upper airway Hypertension Pulmonary HTN Retrognathia/overjet Nasal obstruction Decreased oxygen saturation S3 heart sound(congestive heart failure) Lower extremity edema |
8.5 Cardiovascular
From the cardiovascular point of view, the consequences of OSA, hypoxemia, and intrathoracic pressure changes produce intermediate mechanisms as sympathetic activation, endothelial dysfunction, hypercoagulability, inflammation, oxidative stress, and metabolic dysregulation, which finally develop into cardiovascular diseases, systemic hypertension, heart failure, arrhythmia, stroke, myocardial ischemia, and even sudden death (Fig. 8.3).
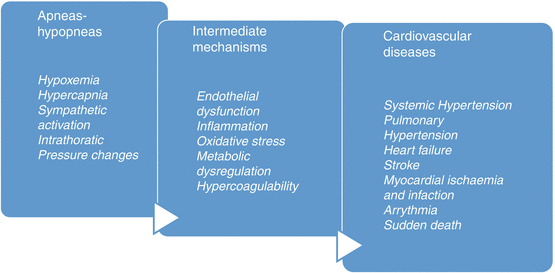

Fig. 8.3
Pathological association between OSA and cardiovascular diseases
8.6 Neurocognitive
OSA is associated with variable degrees of neurocognitive impairment. Traffic accidents and work performance deficits are surrogate markers of these neurocognitive impairments in the OSA population that may be reported in clinic. OSA produces negative effects on inductive and deductive reasoning, attention, vigilance, learning, and memory. OSA usually is manifested by impaired judgment, slowed reaction time, impaired learning, and poor working memory, compromised driving, and poor performance at work. Patients with OSA often complain of difficulty staying on task at work, falling asleep inappropriately at their work space, and memory trouble causing professional difficulties [12].
There also seems to be an association between sleep health and mood disorders. Studies relate risk for the development of depression in patients with OSA and also found a dose-response association. However, other studies have not found a relationship between depression and OSA, particularly in men [1, 11].
8.7 Metabolic and Endocrine
OSA also affects the metabolic profile, particularly in moderate-to-severe disease. The adverse impact of the OSA on glucose homeostasis, lipid metabolism, and fatty liver disease suggests that OSA should be considered as a potential component of metabolic syndrome [1, 11, 12].
Among the causes of metabolic syndrome in OSA patients, alterations in the hypothalamic-pituitary-adrenal axis, aberrant sympathetic activation, induction of certain adipokines, an increased inflammation/oxidative stress and altered glucose metabolism has been described. Investigators found a significant reduction in insulin sensitivity in patients with OSA, and this reduction in insulin sensitivity correlated with severity of the disease, and the correct treatment of apneic events improves glycemic control [7, 11].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






