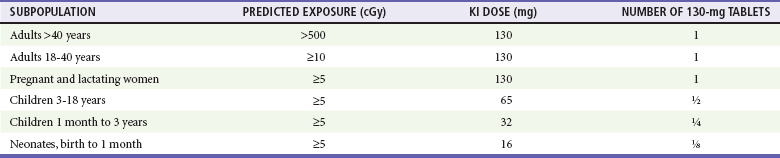
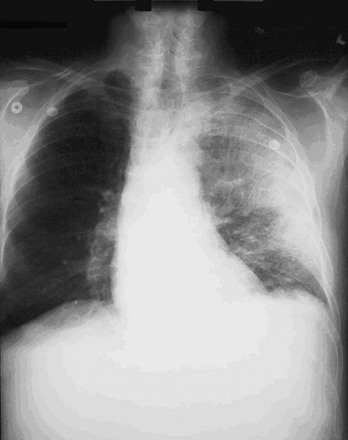
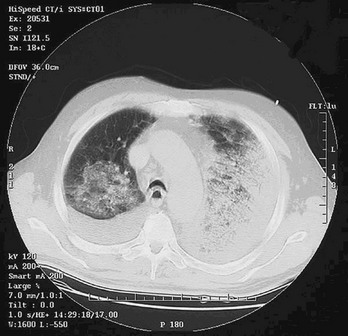
Chapter 194 The results of an attack with WMD, although admittedly of low probability, are potentially catastrophic. According to a World Health Organization estimate, 50 kg of anthrax spores aerosolized above a city of 5 million people would result in 100,000 deaths, with an additional 150,000 people seriously infected. The cost of managing 100,000 cases of anthrax exposure is estimated at between $6.4 and $26.2 billion.1 Given these considerations, most authorities believe that preparedness for such threats is a priority. The use of WMD has been predominantly by the military during times of conflict. In recent history, however, the use of these agents has taken an ominous turn. Nonaffiliated groups have begun using WMD directed at civilians to achieve political ends. The Bhagwan cult sprayed salad bars in Oregon with Salmonella in an attempt to influence an election in 1984.2 The Aum Shinrikyo used the nerve agent sarin in an unsuccessful 1994 assassination attempt on three judges in Matsumoto, Japan. This same group used sarin again in the 1995 Tokyo subway attack that killed 11 people.3 The United States experienced multiple anthrax hoaxes during 1997 and 1998, motivated by personal or political agendas. Terrorists initiated an actual anthrax attack using the U.S. mail in 2001 that resulted in 11 deaths. No one has yet used radiologic or nuclear devices in a successful mass terrorist attack, but at least one attempt has occurred. In addition, several highly radioactive sources have been stolen from U.S. medical facilities, and a Russian dissident, Alexander Litvinenko, was assassinated with a radiologic agent (polonium-210) in 2006. Many agents are potential candidates for weaponization, and some represent a substantial risk (Box 194-1). Management strategies for patients exposed to WMD are frequently similar to strategies for hazardous materials exposure. However, several features associated with WMD make these events unique (Box 194-2). Additional knowledge and skills are required in the evaluation and treatment of WMD victims. These plans represent only one small part of an overall comprehensive emergency management strategy for all hazards (see Chapter 193). Names of departments, bureaus, and agencies that can assist with planning and response to WMD events are listed in Table 194-1. Table 194-1 Resources and Contacts for Planning and Response to Events Involving Weapons of Mass Destruction Instead, simple radiologic devices, such as those used by hospitals for radiation therapy, are thought to be the source of choice. These sources are plentiful. They do not detonate on their own and give no warning of their presence unless they are dispersed by a conventional explosive (radiologic dispersal device). Thefts of radiotherapy sources have occurred in the United States. Accidental dispersion from a stolen hospital therapy source in Brazil resulted in the screening of 112,000 people for contamination. A total of 249 people were found to be exposed, 4 of whom ultimately died.2 Placement of such a device at an information kiosk in a crowded mall during a busy holiday shopping season would silently expose countless persons to significant radiation. Ionizing radiation, regardless of its type, causes injury at the cellular level, usually by damaging DNA. Rapidly dividing cells are the most sensitive. Patients have symptoms within hours to days, depending on the dose. Common syndromes associated with radiation exposure include dermal burns, bone marrow failure, and gastrointestinal dysfunction (e.g., vomiting and gastrointestinal bleeding) (see Chapter 146). A U.S. Department of Homeland Security task force developed an expert consensus document on medical treatment of radiologic casualties, and the results were subsequently published in the peer-reviewed literature.4 Contaminated patients are more challenging, and early involvement of the radiation safety officer is critical. This individual evaluates the degree of the victim’s contamination and monitors radioactivity levels throughout the decontamination process. Internally contaminated patients present a therapeutic challenge because they have radioactive material inside their bodies (e.g., lungs and gastrointestinal tract) or incorporated into their cells. They should be placed in an isolation room, where all secretions and body fluids can be collected. Various medications are available for administration to internally contaminated patients and can limit uptake or facilitate removal of certain radioactive elements. These medications include Radiogardase (Prussian blue) for cesium and thallium ingestions and diethylenetriaminepentaacetic acid (DTPA) for plutonium exposure. Health care providers can receive assistance by calling the Radiation Emergency Assistance Center/Training Site (REAC/TS; http://orise.orau.gov/reacts) at 865-576-3131 (emergency number: 865-576-1005). Externally contaminated victims have radioactive material on their skin or clothing and are decontaminated by removal of clothing and washing with soap and water. Washing by protected personnel should continue until monitoring by the radiation safety officer demonstrates the absence of radioactivity. If wounds are present, they are decontaminated first. After the wounds are covered with a sterile, waterproof dressing, the remaining skin is washed. Hospitals must be prepared to decontaminate patients because historical data suggest that up to 80% of patients do not receive this intervention before arrival.2 Decontamination before hospital entry is crucial because these individuals can expose caregivers to radiation and contaminate the entire hospital through the ventilation system. Removal of clothing and covering of the head with a surgical cap can reduce contamination by 80% to permit stabilization in the decontamination unit, but complete decontamination should occur before exposure of unprotected staff if the patient’s medical condition permits. Initial triage of radiation casualties is based on their overall pathologic condition, not on exposure.5 Even patients who have received a lethal dose of radiation do not die immediately as a consequence of the ionizing exposure. Therefore, a patient in acute distress from a myocardial infarction or urosepsis would be triaged ahead of a radiation victim with stable vital signs, regardless of the dose received. If a radiation casualty also suffers a severe injury or illness, immediate intervention is required. Most of the immediate morbidity and mortality associated with a radiologic dispersion device is related to traumatic injuries from the explosion and not to radiation exposure.4 Although many radioactive elements are candidates for use in a terrorist attack, 131I and related isotopes deserve additional discussion because of heightened interest. 131I is found only after a nuclear detonation or in reactor fuel rods. Although it is not impossible, the probability that terrorists could tap either of these sources is very small. The use of 131I in a radiologic dispersal device is unlikely because of its short half-life (8 days). Even if such a device could be made, it is extremely unlikely that the radiologic dispersal device could disperse sufficient radioactive material to pose an immediate health hazard.6 Given these facts, the probability that any significant exposure of the population (especially children) to 131I will occur is equally small. The large number of childhood thyroid cancers that occurred after the accident at the Chernobyl nuclear power plant resulted, to a significant degree, from situations that will not occur in the United States. These include delayed reporting of a breach in the reactor containment vessel preventing timely evacuation of all exposed populations, failure to effectively quarantine contaminated milk and vegetables, and significant iodine deficiency in the exposed population.7 The risk to children in communities surrounding the Fukushima nuclear power plant is also an issue that will require long-term monitoring. Nonetheless, concern about treatment to prevent thyroid cancer after potential exposure to 131I remains. Current recommendations for treatment with potassium iodide, which blocks uptake of 131I by the thyroid, are listed in Table 194-2. Caveats for use of this table include increasing the amount of potassium iodide for adolescents approaching 70 kg to the adult dose (130 mg) and monitoring thyroid-stimulating hormone and free T4 levels in neonates when possible. Nonpregnant adults older than 40 years are unlikely to benefit from this intervention. Patients exposed to biologic agents usually present with vague symptoms associated with an influenza-like illnesses. Unless a biologic attack is announced or suspected, the emergency department staff may not realize that they are treating victims. Indeed, it is not always possible to distinguish natural occurrences from engineered outbreaks of diseases. Examples of non-terrorist outbreaks of anthrax include cutaneous disease in intravenous heroin users in Europe and an outbreak of cutaneous anthrax in Bangladesh in 2010 with more than 400 cases. Because of the challenges in identifying the true etiology of acute events, personnel should be vigilant and at least consider the possibility, especially when warning signs are present (Box 194-3). For example, large numbers of patients suddenly presenting with “the flu” not during influenza season should cause concern. For these reasons, health surveillance will be paramount in identifying agents and potential sources. The emergency department should have a working relationship with local and state health departments as well as with local law enforcement and stay apprised of Centers for Disease Control and Prevention (CDC) and Department of Homeland Security guidelines. Several infectious agents with potential for use as biologic weapons can spread in a hospital environment. Examples include Ebola and smallpox.2,8 Hospitals need protocols for PPE and patient isolation to ensure a safe environment.9–12 Fortunately, such protocols are similar to those applied to other infectious diseases (Box 194-4). Implementation of such precautions is credited with halting of the in-hospital spread of the Ebola virus in the 1995 Zaire outbreak. Decontamination is not a priority unless the exposure is immediate. Standard (universal) precautions are generally sufficient, and special suits (e.g., levels A, B, and C) are unnecessary.13 Whereas the CDC lists six Category A (high threat) agents (www.bt.cdc.gov/agent/agentlist-category.asp), this chapter focuses on three biologic agents—anthrax, plague, and smallpox—that represent the greatest interest.14 Russia and the United States have developed anthrax into a biologic weapon. The effectiveness of this agent was clearly demonstrated by two events: an accidental release of spores from a biologic weapons facility in the former Soviet Union town of Sverdlovsk in 1979 and the intentional distribution of anthrax spores through the mail along the eastern seaboard of the United States in 2001. After the Sverdlovsk release, at least 66 people died downwind from the compound during the next several weeks, and animal cases of anthrax were reported 30 miles away.15,16 The ability of non–state-sponsored terrorist groups to develop anthrax as a weapon is uncertain. The Japanese organization Aum Shinrikyo made several attempts to disperse anthrax throughout Tokyo without success.1 The individual believed responsible for the U.S. anthrax attack was not a foreign national. This is consistent with the fact that the strain of anthrax used in the attack (Ames strain) was developed by the U.S. government. Inhalational anthrax is the most lethal form of the disease and is caused by inhalation of spores into the lungs. The mortality rate was thought to exceed 90%. However, data from the 2001 anthrax exposure call this figure into question (5 deaths in 11 cases). Although the actual mortality rate is unknown, it is probably in the 50% range.17,18 The minimum number of spores required to produce disease is unknown. The original number quoted in the literature, 104 spores, appears high given recent experience.19 After phagocytosis by macrophages, the spores germinate and are transported to the tracheobronchial lymph nodes, where the bacteria multiply. During 2 to 10 days, patients have an influenza-like illness, with malaise, fever, and nonproductive cough. This initial phase can be delayed for more than 1 month in some patients. Within 24 to 48 hours, abrupt deterioration occurs, with overwhelming sepsis, shock, hemorrhagic mediastinitis, dyspnea, and stridor. A chest radiograph obtained at this time may show a widened mediastinum and hilar adenopathy, but typical radiographic findings are not dramatic and could be missed (Fig. 194-1). Computed tomography scanning of the chest is more sensitive and should be performed if the disease is suspected. Bloody pleural effusions can also occur, and examination of the lung fields frequently reveals consolidation. This can easily be confused with pneumonia (Fig. 194-2). Death usually results within 3 days, and 50% of patients have hemorrhagic meningitis. Human-to-human transmission has not been reported with inhalational anthrax.
Weapons of Mass Destruction
Perspective
ORGANIZATION
WEBSITE
TELEPHONE
Radiation Emergency Assistance Center/Training Site (REAC/TS)
http://orise.orau.gov/reacts
Daytime: 865-576-3131
Emergency: 865-576-1005
State and local health departments
www.statepublichealth.org
www.cdc.gov/other.htm#states
Centers for Disease Control and Prevention (CDC)
www.bt.cdc.gov
800-CDC-INFO
Federal Bureau of Investigation (FBI)
www.fbi.gov
Federal Emergency Management Agency (FEMA)
www.fema.gov
800-621-FEMA
U.S. Army Medical Research Institute of Chemical Defense
http://chemdef.apgea.army.mil
Nuclear and Radiologic Devices
Biologic Weapons
Anthrax
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Weapons of Mass Destruction
Only gold members can continue reading. Log In or Register to continue