CHAPTER 47 Ventricular Assist Device Therapy in Advanced Heart Failure—State of the Art
THE BURDEN of congestive heart failure (CHF) continues to grow, with more than 500,000 new cases annually. Fortunately, increased understanding of the disease process, and advances in pharmacologic and pacemaker-based therapies have led to significant improvements in survival for patients with CHF over the past 2 decades.1 For example, 1-year mortality in subjects with NYHA class IV CHF participating in clinical trials investigating ACE-inhibitors, β-blockers and aldosterone antagonists has decreased from approximately 50% in 1986 to less than 15% in 2002.2–4 However, many patients with advanced CHF continue to have significant symptoms despite these interventions. For these patients, prognosis remains very poor with 1-year mortality rates approaching 75%.5
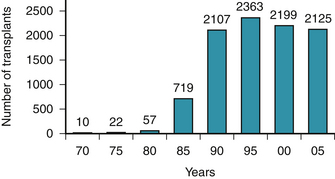
When CHF is refractory to medical therapy, and in appropriate candidates, cardiac transplantation is the most effective treatment modality. Refinement in techniques and immunosuppressive regimens has produced 1-year survival rates of approximately 90%.6,7 However, the number of transplants performed annually in the United States has plateaued at 2200 because of a limited number of donor organs (Fig. 47-1), and many patients die while waiting for a transplant.8
In the last 15 years, left ventricular assist devices (LVADs) have emerged as a method to prolong the survival of these patients as they wait for a suitable donor heart. By mechanically unloading the failing heart and improving systemic end-organ perfusion, LVADs can reverse the systemic abnormalities seen in advanced CHF, improving survival and quality of life. Advances in design and clinical experience have significantly reduced the morbidity and mortality associated with LVADs, and have led to the continual spread of this technology.9 Further advances are currently under evaluation and promise to make LVADs available to a greater number of patients. Importantly, use of LVADs is no longer limited to subjects waiting for heart transplants, but is now approved to treat end-stage heart failure refractory to medical therapy in patients not suitable for transplantation because of age and/or comorbidities. This review discusses the evolution of LVAD devices, the current uses of this therapy, and its potential future applications.
Background
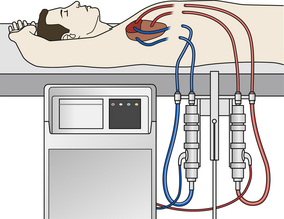
Mechanical circulatory support devices were initially developed in the 1960s as a means to assist patients who could not be weaned from cardiopulmonary bypass following cardiac surgery. Dr. Michael E. DeBakey implanted the first device in 1963 to treat cardiogenic shock following aortic valve surgery. Although the patient did not survive to hospital discharge, this operation proved the feasibility of mechanical circulatory assistance.10 Through the 1960s and 1970s, researchers developed several devices for investigational use. These “early” LVADs caused significant hemolysis and had high rates of bleeding and infection because of their size and extracorporeal location. Furthermore, patients with otherwise good clinical recovery remained significantly limited in their mobility because of the large power consoles (Fig. 47-2).
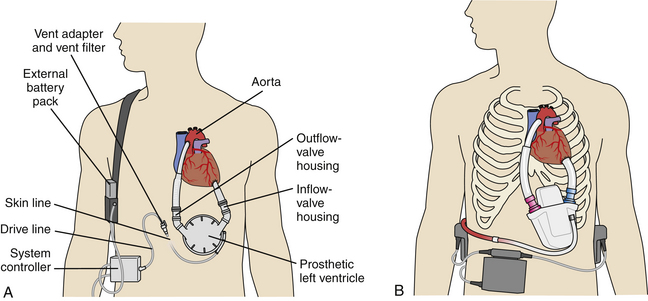
Limitations of the early devices, however, provided the impetus for development of an intracorporeal LVAD powered from an external source through a percutaneous driveline. Situating the pump inside the body provided patients with greater independence, allowed discharge from the hospital, and substantially improved quality of life. In addition, outpatient LVAD management led to significant cost savings over prolonged inpatient stays.11 Consequently, the Heartmate 1000 IP (Thoratec Corp., Pleasanton, Calif.) received FDA approval as a bridge-to-transplantation device in 1994. The power console of this pneumatic LVAD was smaller than that of earlier pumps, but its size remained a significant limitation. Further research produced an electric power source connected to a wearable battery pack. An LVAD using this technology, the Heartmate Vented Electric (VE) (Thoratec Corp.), was approved by the FDA in 1998 (Fig. 47-3).
LVAD as Bridge to Transplantation
Currently, there are two LVADs approved for bridge to transplantation: the Heartmate I and the Novacor (World Heart Corp., Ottawa, Ontario, Canada). Both are surgically implanted in a pocket in the abdominal wall (see Fig. 47-3). An inflow conduit is attached to the left ventricular apex and an outflow conduit is anastomosed to the ascending aorta. Bioprosthetic valves ensure unidirectional blood flow. The blood is expelled by electromagnetically powered pusher plates that contract a polyurethane diaphragm and provide pulsatile flow to the aorta. The devices can pump blood at rates up to 10 L/min. Each device is connected to a wearable battery pack through a percutaneous line that also houses the venting system.
In patients refractory to medical therapy, LVAD support leads to improved renal function, right heart function, and overall physical conditioning.11,12 Multiple studies have shown that LVADs can be successfully used as a bridge to transplantation,13–16 with one prospective series showing an increase in survival to transplant from 33% to 71% with use of the Heartmate LVAD (when compared with historical controls managed with medical therapy alone).15 By implanting an LVAD in these patients, surgeons reduce their subsequent operative risk for transplantation, making them better transplant candidates and improving their posttransplant outcomes.15,16
Although most commonly implanted into patients with symptoms of Class IV CHF that are refractory to medical therapy, intracorporeal LVADs have also been used for the treatment of post–cardiotomy shock, myocardial infarction complicated by cardiogenic shock, myocarditis, and primary graft failure following cardiac transplantation.17–20 The following criteria should generally be met before consideration of LVAD implant: clinical evidence of impaired end-organ perfusion with cardiac index less than 2 L/min/m2, pulmonary capillary wedge pressure greater than 20 mm Hg, and systolic blood pressure less than 80 mm Hg despite maximal medical support including inotropes and an intra-aortic balloon pump if indicated.21 Although it is important to meet these criteria, LVAD implantation needs to be performed before extensive end-organ damage for maximal chance of success.
Adverse Events
Table 47-1 shows the most commonly encountered adverse events in patients who receive LVADs as a bridge to transplant. Different complications are seen in the early period following LVAD implantation and the later period following prolonged use.
Table 47–1 Adverse Events in Patients with the Heartmate VE When Used as a Bridge-to-Transplantation
| Event | Frequency |
|---|---|
| Bleeding | 48% |
| Infection | 48%-55% |
| Neurologic event | 10%-27% |
| RV failure | 7%-11% |
| Thromboembolism | 12% |
| Device failure | 12.8% |
Adapted from references 6, 15, and 25.
The two major perioperative complications are bleeding and right ventricular (RV) failure. Bleeding causes significant morbidity and mortality, and often requires exploration of the surgical site. The degree of RV failure is difficult to predict before LVAD implantation, although the presence of elevated central venous pressure (CVP), high pulmonary vascular resistance, and significant RV dilation raise concern that additional RV support may be required postoperatively. The importance of the RV in the management of LVAD patients cannot be underestimated. With a functioning LVAD in place, cardiac output is dependent on the ability of the RV to provide sufficient preload to the left side of the heart. Postsurgical RV dysfunction can compound underlying contractile weakness and cause right heart failure, which is correlated with worse outcomes.22 Treatment consists of pharmacologic augmentation of RV contractility and reduction of pulmonary vascular resistance (see “Management of Acute Complications” later). Occasionally, refractory RV failure dictates placement of a right ventricular assist device (RVAD).
With intermediate and long-term use, the most common complication is infection, occurring in up to 55% of patients.23–25 The relative immunosuppression of critical illness and the presence of large amounts of foreign material leaves LVAD patients particularly susceptible to infectious complications. Infections can occur in the device pocket or surrounding surgical area, along the percutaneous drive lines and inside the device itself. Prevention efforts, including enhanced prophylactic antibiotic regimens, new surgical techniques, improved drive line design, and attention to drive line management are critical in reducing the overall infection rate.26
The risk of thromboembolism and neurologic events has been significantly reduced through the introduction of the Heartmate LVAD. The Heartmate I has a unique, textured design that promotes the formation of an endothelial layer on its blood-contacting surfaces. This “natural” surface has low thrombogenicity, and the Heartmate therefore does not require systemic anticoagulation.27 With other devices, thrombotic complications remain prevalent and necessitate anticoagulation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree