Venous Thromboembolism
Gerard Fulda
Mark Cipolle
I. Deep Venous Thrombosis (DVT)
Definition. DVT refers to any clot (obstructing or nonobstructing) in any deep venous system, including the upper extremity and the calf.
Pathophysiology. The mechanism behind clot development relates to Virchow’s Triad: Stasis, injury to the vessel wall, and hypercoagulability. Stasis causes interruption of normal laminar flow allowing platelets to come in contact with endothelium; interruption of endothelial integrity exposes the extracellular matrix to platelets; hypercoagulability relates to excess circulating procoagulant factors +/− diminished anticoagulant factors. In trauma, most evidence points to intimal injury as the initial inciting event, followed by platelet adhesion, activation of the procoagulant system and release of thrombin. Hence, direct thrombin inhibitors (DTIs) play a strong role in prevention.
Epidemiology
Incidence. DVT affects >2.5 million people each year in the United States. This is likely an underestimate as many cases go unrecognized by patient and physician. Series have shown the incidence of DVT to be as high as 65% in the untreated major trauma patient.
Risk factors. In general, these include age >75, immobilization, acute infectious disease, cancer, general anesthesia, major surgery, estrogen therapy, pregnancy, prior DVT, congestive heart failure, malignancy, hypercoagulable state, and tissue trauma. Specific to trauma, risk factors include advanced age, lower extremity fracture, spinal cord injury, head injury (AIS >3), ventilator days >3, shock, multiple blood transfusions, surgery, fracture of the femur or tibia, complex pelvic fracture, venous injury, immobility, and spinal cord injury. Scoring systems have been developed to assess risk factors and better estimate the risk of DVT or pulmonary embolism (PE) (Table 16-1).
Location. DVT can occur in any deep venous system.
Calf vein thrombosis. Calf vein thrombosis is a clot localized to one or more of the three major named vessels below the knee. Untreated, these can propagate proximally in up to 23% of cases; however, they can also resolve without complications. Treatment is therefore controversial; however, follow-up duplex examination to rule out propagation should be performed.
Iliofemoral vein thrombosis. Most common site in the trauma patient. Findings may be subtle. A classical presentation is phlegmasia cerulea dolens (PCD)—painful, swollen, bluish leg. More common on the left. Higher incidence in patients who have had femoral venous lines.
Upper extremity thrombosis. The most common cause of upper extremity DVT is subclavian vein catheterization, rates, reported up to 46%. The incidence of PE may be as high as 12%. Treatment is extrapolated from treatment regimens for lower extremity DVT as no good trials exist for this entity specifically. The risk is increased with the length of time the catheter is in place as well as larger or malpositioned catheters.
Pelvic vein thrombosis. Missed by commonly used screening modalities.
Table 16-1 DVT Risk Factor Categories
A. Risk factors:
- – Age 40 years
- – Injury severity score (ISS) >9
- – Blood transfusion
- – Surgical procedure lasting ≥2 h
- – Lower extremity fracture
- – Pelvic fracture
- – Spinal cord injury (SCI)
- – Immobilization
- – Pregnancy
- – Estrogen therapy
- – History of DVT/PE
- – Malignancy
- – Hypercoagulable state (e.g., AT III deficiency)
- – Extensive soft tissue trauma
- – Congestive heart failure (CHF)
B. High risk factors:
- – Age >50 years
- – ISS ≥15
- – Femoral central venous catheter in trauma resuscitation
- – AIS ≥3 (any body region)
- – Glasgow coma score (GCS) ≤8
- – SCI
- – Pelvic fracture
- – Femur or tibia fracture
- – Venous injury
C. Very high risk factors:
- – SCI
- – AIS-Head/neck ≥3 + long bone fracture (upper or lower)
- – Severe pelvic fracture (posterior element) + long bone fracture (upper or lower)
- – Multiple (≥3) long bone fracture
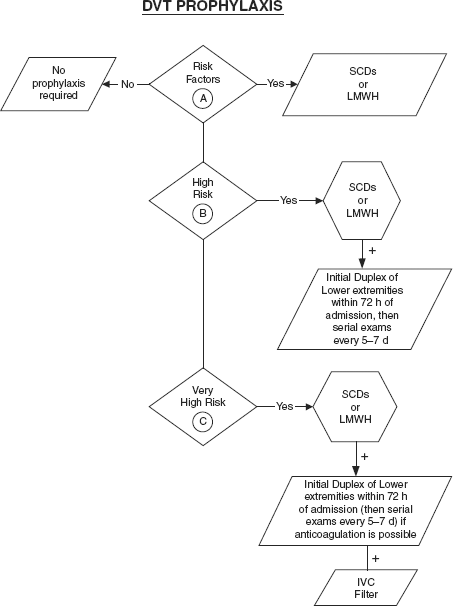
SCD, sequential compression device; LMWH, low molecular weight heparin.
Modified from Clinical Management Guidelines, Deep Venous Thrombosis Prophylaxis. Division of Trauma and Surgical Critical Care. Hospital of the University of Pennsylvania. Philadelphia, PA, 2000.
Complications. DVT is a major cause of morbidity and mortality.
Local complications. Although rare, local ramifications of DVT include phlegmasia cerulea dolens (edematous, bluish extremity) or phlegmasia alba dolens
(blanching “milk leg”) with potential ulceration, loss of arterial flow, and/or resultant venous gangrene.
Pulmonary embolism. Incidence of pulmonary embolism after trauma varies with population, injury pattern, use of prophylaxis, and method of detection. Overall, 1% to 2% of patients with DVT after trauma will have a pulmonary embolism which carries a 30% to 50% mortality risk.
Postphlebitic syndrome. Venous valves in the lower extremities are destroyed by clot formation when DVT occurs. After the clot dissolves, valvular competence is permanently lost in the affected segment of vein. As a result, nonpitting edema, swelling, discoloration, and pain frequently occur, which can progress to venous hypertension and venous stasis ulceration. The incidence of postphlebitic changes at 12 years post-DVT approximates 28%. Severe sequelae occur in less than 6%.
Diagnosis.
Clinical manifestation. Subjective complaints of pain or swelling of the affected extremity are rare. In fact, only 40% of patients have any clinical signs or symptoms. The classic syndrome of calf discomfort, edema, venous distension, and pain on dorsiflexion of the foot (Homans’ sign) is seen in less than 30% of patients.
D-dimer. D-dimers are a breakdown product of fibrin and should be elevated when venous thrombosis is present. Fibrin degradation products are elevated in
the first 48 hours after traumatic injury. Therefore, there is little utility in using admission d-dimer to diagnose DVT in the trauma patient.
Compression ultrasound. Duplex ultrasound (DUS) combines real-time B-mode ultrasound with pulsed Doppler capability. In symptomatic patients, the sensitivity and specificity are greater than 95%; thus, DUS is the most commonly performed test for the detection of infrainguinal DVT. The addition of color flow imaging reveals physiologic flow characteristics and may be useful in technically challenging examinations. Whether ultrasound is performed with flow assessment or not, compression of the vein along its length is the key aspect in evaluation for DVT. Noncompressibility of the vein is the primary diagnostic criterion for acute DVT. Other DUS findings include an echogenic thrombus within the vein lumen, venous distension, complete absence of spectral or color Doppler signal from the vein lumen, or loss of flow phasicity, response to Valsalva, or augmentation. Limitations of DUS include patient characteristics (obesity, edema, or tenderness), inability to ultrasound through various devices (casts, etc.) or compression of a vein by perivenous pathology (tumor, hematoma). In addition, DUS is unreliable in evaluating iliac veins.
Surveillance ultrasound. Routine weekly surveillance of patients at high risk for DVT will increase the yield of discovering asymptomatic DVT. However, there is no strong evidence that the practice decreases PE rates and the cost-effectiveness of this approach has not been demonstrated. Routine use of surveillance is not recommended (CHEST guidelines). Ultrasound is indicated in the evaluation of symptomatic patients.
Contrast venography. While contrast venography (CV) remains the gold standard for diagnosis of DVT, it is rarely used due to the accuracy of noninvasive testing. CV is invasive and requires intravenous injection of contrast dye with the risks associated; however, it is considered to be 100% sensitive and specific. Diagnosis requires a constant intraluminal filling defect in two views or an abrupt cutoff of a deep vein.
CT venography (CTV). CTV uses venous phase contrast to directly visualize the inferior vena cava, pelvic veins, and lower extremity veins. It can be timed immediately after a CT pulmonary angiogram (CTPA) or used alone; however, when combined with CTPA, many series report a higher IV contrast dose is necessary for accurate visualization of the vessels. CTV can be plagued by artifact, such as from orthopedic hardware, or poor venous enhancement. The sensitivity and specificity are 89% to 100% and 94% to 100%, respectively. Some advantages of CTV are the readily available of CT scan in most hospitals at off-hours and the ability of a single study to diagnose both PE and DVT.
Prophylaxis.
Upper extremity DVT: Early removal of any indwelling central venous catheter is the best way to prevent upper extremity DVT. Chemoprophylaxis is not recommended to prevent upper extremity DVT.
Lower extremity DVT: As trauma patients are at high risk for the development of VTE, many centers have developed standardized algorithms for prophylaxis. These forms include early ambulation, mechanical devices, such as sequential pneumatic compression devices (PCDs), and pharmacologic therapy, such as low molecular weight heparin (LMWH). All major trauma patients should have some form of DVT prophylaxis. Prophylaxis should begin at the time of admission. If chemoprophylaxis cannot be initiated due to the risk of bleeding, mechanical prophylaxis should be used until chemoprophylaxis can be initiated. Prophylaxis should be continued at a minimum until the patient is fully ambulatory. In some cases of extensive orthopedic injuries, patients should be discharged on chemoprophylaxis. Traumatic brain injury is not an absolute contraindication to chemoprophylaxis; however, the risk of bleeding needs to be assessed prior to initiating therapy. Patients with small stable intracerebral hemorrhages generally can be started on chemoprophylaxis after 3 days.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







