Agent
Dose
Mechanism of action
Length of therapy
Notes
Octreotide
50 μg bolus IV; then 50 μg/h continuous infusion
Somatostatin analogue
3–5 days
May result in tachyphylaxis
Most commonly used in U.S.
Somatostatin
250 μg bolus IV; then 250 μg/h continuous infusion
Inhibits release of vasodilatory hormones
3–5 days
Hormones inhibited include glucagon, insulin, serotonin, acetylcholine
Terlipressin
2 mg IV every 4 h; may decrease to 1 mg every 4 h when hemorrhage controlled
Synthetic vasopressin analogue
3–5 days
Longer half-life allows intermittent therapy
Not available in U.S.
Vasopressin
0.4 unit bolus IV; then 0.4–1.0 units/min
Vasoconstricts mesenteric arterioles
3–5 days
Higher risk of extrasplanchnic ischemic side effects
May administer with IV Nitroglycerin to decrease side effect risks
Rarely used in U.S.
Hemostasis Interventions
Emergent endoscopy should be performed within 12 h of presentation in patients suspected of having active gastroesophageal variceal bleeding (Fig. 62.1) [8, 9]. Multiple studies have demonstrated endoscopic variceal ligation is superior to endoscopic sclerotherapy [8]. A meta-analysis of seven studies revealed that ligation therapy compared to sclerotherapy results in a similar rate of hemostasis, but significantly less re-bleeding and lower mortality [10]. Sclerotherapy is more commonly used when variceal ligation is technically difficult or as a rescue procedure.
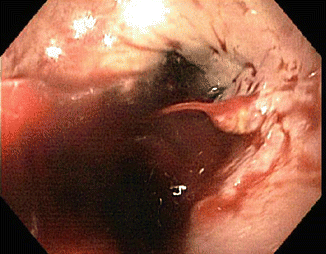

Fig. 62.1
Esophageal varix with active bleeding as seen during EGD
Transjugular Intrahepatic Portosystemic Shunt
Approximately 10–20 % of patients will fail standard medical therapy for acute variceal bleeding [11]. Risk factors for treatment failure in the first 5 days following initial hemostasis include low systolic blood pressure at presentation, active bleeding at endoscopy, bleeding from gastric varices, and higher Child-Pugh grades [6]. Placement of a transjugular intrahepatic portosystemic shunt (TIPS), a stent connecting the portal and hepatic veins, results in a decreased portal pressure gradient and is commonly used as rescue therapy in patients in whom initial hemostasis is not achieved and in patients with recurrent variceal bleeding following initial hemostasis (Fig. 62.2) [12]. One potential complication of TIPS placement is thrombus formation in the stent. The use of coated stents recently has seemed to decrease the rate of this event [11]. The interventional radiologist may also place coils or apply sclerosant to varices directly at the time of TIPS placement (Fig. 62.3 and Video 62.1).
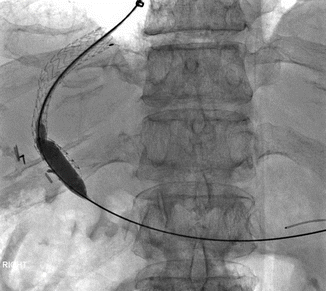
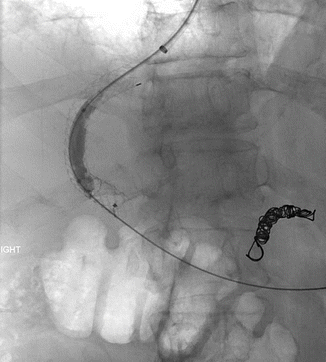

Fig. 62.2
A TIPS stent has been inserted to connect the right hepatic and right portal veins. The stent is post-dilated to ensure adequate flow (Images courtesy of Dr. Bill Majdalany of the University of Michigan)

Fig. 62.3
Coil embolization of the gastric coronary varix seen in Fig. 62.3 has now been performed (Images courtesy of Dr. Bill Majdalany of the University of Michigan)
Antibiotic Prophylaxis
Prevention of complications such as worsening hepatic function, renal dysfunction and bacterial infections may improve survival following an acute bleeding episode [13]. The most common infections include bacteremia, urinary tract infections and spontaneous bacterial peritonitis [14]. Antibiotic prophylaxis in the peri-bleed period has been shown to decrease all-cause mortality by 20 % and also decreases overall re-bleeding episodes [15]. Cephalosporins and quinolones are the most effective therapies. Antibiotics should routinely be administered to patients with acute variceal hemorrhage [9].
Evidence Contour
While certain therapies are proven in variceal bleeding, others are performed according to expert recommendations or guidelines and are more controversial. A discussion of these management strategies will follow.
Red Blood Cell Transfusion
The threshold for transfusion in actively bleeding patients has been debated throughout the years. In 2013, a landmark trial demonstrated the utility of a restrictive transfusion protocol in patients with suspected upper gastrointestinal bleeding. A restrictive protocol (transfusion for hemoglobin <7.0) as compared to a liberal protocol (transfusion for hemoglobin <9.0) resulted in a lower 45-day mortality rate (5 % versus 9 %, p = 0.02), lower risk of further bleeding, lower likelihood of requiring rescue therapies, and a lower rate of complications including pulmonary edema and transfusion reactions. The authors postulated that the increase in mean hepatic venous pressure gradient in the liberal group likely contributed to these findings [16]. Of note, only around a quarter of the patients in this study had variceal bleeding. Furthermore, patients with poorly defined “massive exsanguinating bleeding” were excluded from this study. In general, a goal hemoglobin level of 7.0 is reasonable in patients with variceal bleeding for these reasons, although in the setting of massive exsanguination, red blood cell transfusion should not be directed at a hemoglobin level but should be based on the clinical situation.
There have been no randomized, controlled trials evaluating the administration of crystalloid compared to colloid fluids in patients with acute variceal bleeding. However, in other patient populations with hemorrhagic shock, colloid fluids such as albumin or hetastarch have no advantage compared to crystalloids [17]. In the cirrhotic patient population with variceal bleeding, it is reasonable to transfuse red blood cells if the hemoglobin is less than 7.0 and crystalloid fluids when the hemoglobin is 7.0 or greater and the patient has signs of hypoperfusion.
Correction of Coagulopathy
Cirrhosis often results in decreased levels of all clotting factors, dysfibrinogenemia, and thrombocytopenia, all of which can contribute to ongoing bleeding. In addition, multiple transfusions can result in a dilutional coagulopathy. Frequent monitoring of prothrombin time, platelet count and fibrinogen levels is warranted.
Unfortunately, prothrombin time has not been shown to correlate well with clinical bleeding in cirrhotic patients [18, 19]. No randomized trials of Vitamin K supplementation in patient with variceal bleeding have been performed, and routine use is neither recommended nor discouraged [20]. Providers should aim to avoid over-transfusion solely to correct numerical lab values as this will likely result in higher portal venous pressures and possibly more bleeding. Recommendations for a threshold at which to transfuse fresh frozen plasma, cryoprecipitate and platelets vary and become even more complicated in the setting of cirrhosis. While trauma literature supports the use of a “massive transfusion protocol,” which entails transfusing packed red blood cells, fresh frozen plasma and platelets in a 1:1:1 ratio [2], this strategy has not been tested in a randomized trial of cirrhotic patients with variceal bleeding. It is unknown if this is the correct resuscitation approach in our patient population, and it should not be universally applied.
Recombinant factor VIIa, a product first available for patients with hemophilia-related bleeding, has been considered as an agent that could halt gastrointestinal bleeding in cirrhotic patients by activating the extrinsic pathway and increasing thrombin production. While recombinant activated factor VIIa has been shown to normalize prothrombin time in patients with cirrhosis and variceal bleeding [21], a Cochrane review that included approximately 500 patients with various stages of cirrhosis and active upper gastrointestinal bleeding from two trials did not find a mortality benefit when this factor was administered in addition to usual care. Furthermore, there was no significant difference in packed red blood cell transfusion requirement [22]. An exploratory analysis from one of the two trials suggested that the subgroup of patients with variceal bleeding, Child-Pugh scores B or C, and more severe coagulopathy may benefit from recombinant factor VIIa [23]. Therapy with recombinant factor VIIa remains controversial in this setting.
Case reports have described success in achieving hemostasis in cirrhotic patients with the administration of Prothrombin Complex Concentrate (PCC), a mixture of coagulation factors II, VII, IX and X. This agent cannot be recommended on the basis of current evidence [24].
Endotracheal Intubation
Elective intubation has not been proven to improve outcomes or prevent aspiration events or pneumonia in patients undergoing endoscopy in the intensive care unit. A retrospective cohort study compared patients with suspected variceal hemorrhage who underwent elective pre-endoscopy intubation and those who did not. All patients had active bleeding or stigmata suggestive of high risk of bleeding on endoscopy. Patients who underwent elective intubation had higher mortality and higher rates of aspiration pneumonia. However, patients with a known aspiration event prior to presentation, respiratory distress, intoxication, or hepatic encephalopathy greater than stage 1 were excluded. Patients who were alert at the time of endoscopy protected their own airways better [25].
In another retrospective study, pre-endoscopy intubation did not change the likelihood of witnessed aspiration during EGD, new radiographic infiltrates post-EGD, hospital length of stay, or mortality [26], although it may have prevented massive aspiration events. To this date, no prospective randomized trial has been performed to truly demonstrate the utility or lack thereof of pre-endoscopy elective intubation. Intubation prior to endoscopic therapy should not routinely be employed and should be carefully considered for each individual patient with hemodynamics, mental status and respiratory status in mind.
Acid Suppression
Proton pump inhibitor administration following esophageal variceal ligation has been demonstrated to speed the resolution of post-ligation ulcer healing [27], but their use has been challenged [28]. In a retrospective cohort study, prolonged infusion of proton pump inhibitors in addition to octreotide infusion was compared to short term infusion. No difference in transfusion requirements or mortality rate was observed [29]. However longer proton pump inhibitor infusions may have been selectively administered to patients who were more critically ill, biasing the results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







