Fig. 37.1
Axial CT brain demonstrating right putaminal hemorrhage (From Brott et al. [1]. Reprinted with permission from Wolters Kluwer Health, Inc.)
Question
Would early surgical intervention be appropriate in the initial management of this patient?
Answer
Certain patients may benefit from early neurosurgical intervention in the setting of intracerebral hemorrhage [2].
However, the International Surgical Trial in Intracerebral Haemorrahage (STICH) did not demonstrate improved 6-month functional outcome or mortality with the early evacuation of supratentorial hematomas [3]. Furthermore, STICH II did not demonstrate any significant difference in combined death or disability in patients undergoing evacuation of supratentorial hematoma volumes of 10 to 100 cc without intraventricular hemorrhage (IVH) when compared with the maximal medical management group [4]. However, patients with cerebellar hemorrhages > 3 cm with 4th ventricular effacement and/or hydrocephalus should undergo urgent surgical intervention given elevated morbidity and mortality with conservative management in this group [2, 4, 5]. Younger patients with rapid neurological decompensation secondary to hematoma expansion, or with underlying lesions such as aneurysms, AVMs and tumors may also be surgical candidates [2, 4, 5]. The Minimally Invasive Surgery Plus Recombinant Tissue-Type Plasminogen Activator for Intracerebral Hemorrhage Evacuation II (MISTIE II) trial demonstrated a reduction in perihematomal edema and a trend towards improved outcomes in the hematoma evacuation group [6]. The MISTIE III trial is currently in progress. Phase 3 of Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III) IVH trial is a randomized control study, which aims to evaluate clearance of clot and outcome in patients with large intraventricular hemorrhage receiving scheduled injections of rt-PA through EVD [7]. Study results are expected in the near future.
This patient’s CT scan demonstrates a large right basal ganglia hemorrhage without intraventicular extension. Current guidelines would advocate for maximal medical management [2, 5].
Principles of Management
Clinical Presentation
The onset of acute severe headache, vomiting, seizure, significant hypertension (often > 220 mmHg), and rapid deterioration in consciousness with possible accompanying pupillary dilatation, posturing and abnormal respiratory pattern should provoke a high suspicion for intracerebral hemorrhage (ICH) [2].
Diagnosis
The rapid onset of focal neurological symptoms warrants brain imaging on an emergent basis [2, 5]. CT scans provide a rapid method of evaluating the head for acute pathology, including intracerebral hemorrhage (ICH). Brain CT imaging in the latter often demonstrates intra-axial, focal area(s) of hyperdensity with mass effect and effacement of the surrounding parenchyma [2]. Hemorrhage location(s) provide clues to the underlying pathology. In addition to trauma, multiple etiologies of non-traumatic ICH exist, and include hypertension, vascular malformations, trauma, cerebral amyloid angiopathy, brain neoplasm, infarction, vasculitis, illicit drug-related, bleeding diathesis, and anticoagulation [2, 5]. Hypertension is the most common cause of non-traumatic ICH. Typical locations suggestive of a hypertensive bleed include the basal ganglia (most commonly putamen) and thalamus, as well as the pons and cerebellum [2, 5]. Hematoma volume can be estimated using the ABC/2 method (Fig. 37.2) [9]. Large hematoma volumes and evidence of heterogeneous attenuation are predictive of subsequent hematoma expansion [1, 2, 5, 10].
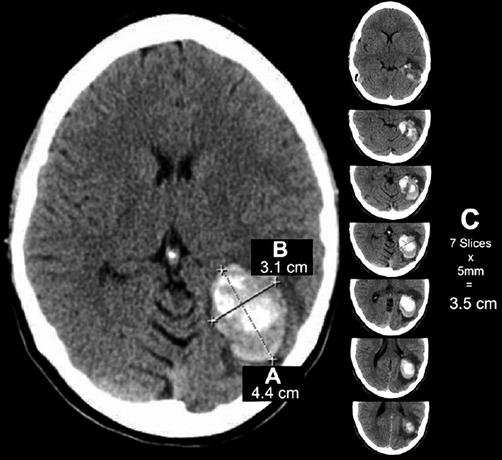

Fig. 37.2
Example of ICH calculation using ABC/2 formula. (a) Greatest hemorrhage diameter by CT. (b) Diameter 90° to A. (c) The approximate number of CT slices with hemorrhage multiplied by slice thickness (From Beslow et al. [8]. Reprinted with permission from Wolters Kluwer Health, Inc.)
Acute Management
In addition to emergent brain imaging, initial laboratory assessments should include coagulation parameters and toxicology screen, with elevations in the former requiring rapid normalization with appropriate reversal agents. Contrast-enhanced head imaging, often CT angiography, can provide additional information about the presence of underlying vascular malformations, aneurysms, as well as the presence of contrast extravasation within the hematoma [1, 10, 11]. The latter “spot sign” is suggestive of active bleeding and predicts hematoma expansion, as well as likely higher morbidity and mortality [1, 10, 11]. Demchuk et al. found a positive predictive value of 61 % for hematoma expansion in the presence of the spot sign [10]. Exacerbating factors for expansion also include antithrombotic and anticoagulation therapy, as well as persistent hypertension, and therefore aggressive management with anticoagulation reversal and antihypertensive therapy is warranted [1, 2, 5, 10].
Serial CT head imaging, usually within 4–6-h, is critical for the evaluation of hematoma expansion and obstructive hydrocephalus. Brott and Broderick found 38 % of hematomas expand by 33 % of original volume or at least 6 cc within 24-h of symptom onset, with most reaching significant expansion within 4 h [1]. Intraventricular hemorrhage is a risk factor for development of the hydrocephalus [12]. The initial presence or subsequent development of ventricular system enlargement warrants rapid neurosurgical intervention with external ventricular drain (EVD) placement and drainage of cerebral spinal fluid (CSF) [2, 5, 12].
Airway patency should be monitored carefully. Large or rapidly expanding hematomas, particularly with evidence of obstructive hydrocephalus, often result in depressed mental status and compromised airway protection [2]. Emergent intubation is warranted in these circumstances with utilization of agents such as propofol and lidocaine to blunt potential intraprocedural intracranial pressure crises [2].
Brain herniation is the most dreaded outcome of ICH, leading to increased morbidity and mortality, particularly in the absence of rapid intervention and reversal. Clinical manifestations include the development of new or worsening neurological deficits, including depressed mental status and pupillary abnormalities, thus mandating frequent monitoring of the patient’s neurological exam in acute setting [2, 5]. Rapid intervention is critical and includes elevating the head to 30°, hyperventilation (with rapid sequence intubation in the setting of an unsecured airway), and administration of hyperosmolar therapy [2, 5, 13]. Repeat head imaging is warranted when the patient is stabilized with insertion of an EVD if obstructive hydrocephalus is present [1–5, 11]. Ultimately the patient may require decompressive hemicraniectomy to improve odds of survival, even though the data for functional outcome amelioration amongst survivors is not nearly as robust as for large hemispheric ischemic strokes [2–6].
Natural History
The ICH score is a risk stratification scale using the Glasgow Coma Score (GCS), age, ICH volume, infratentorial location, and presence of IVH to predict 30-day mortality (Table 37.1 and Fig. 37.3) [14]. The tissue at the epicenter of the hematoma undergoes rapid destruction causing clinical neurological insult and is unlikely to be salvaged [15]. Hematoma expansion and perihematomal edema can worsen mass effect and may contribute to further injury and higher mortality. Edema develops early after ICH and peaks up to 2 weeks after onset, although the most rapid increase occurs in first 2 days on MRI imaging [16]. The degree of edema is predictive of poor outcome. Secondary injury as a consequence of blood–brain-barrier damage, inflammation, and reticulocyte lysis can further worsen injury to the surrounding parenchyma [15, 16].
Table 37.1
ICH score for predicting 30-day mortality
Component | ICH score points |
|---|---|
GCS | |
3–4 | 2 |
5–12 | 1 |
13–15 | 0 |
ICH volume (mL) | |
≥ 30 | 1 |
< 30
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |




