A. Anatomic and physiologic changes. Maternal anatomic and physiologic changes occur during pregnancy to ensure enough blood flow is delivered to the uterus. Uterine blood flow occurs through two routes.
1. The uterine arteries are the primary source of blood flow to the gravid uterus. The uterine arteries are branches of the internal iliac artery, also known as the hypogastric artery. Blood flow through the uterine vessels is characterized as high flow through a low-resistance circuit. Uterine blood flow increases throughout gestation. The uterus receives up to 20% of the maternal cardiac output during the third trimester.2 Flow through each uterine artery can reach 225 to 375 mL per minute at term.3
CLINICAL PEARL Up to 20% of the maternal cardiac output goes to the uterus at term, which increases the urgency of treating the hemorrhaging parturient.
2. Ovarian arteries provide up to 15% of uterine blood flow in addition to that provided by the uterine arteries. The ovarian artery originates from the aorta, and after passing through the infundibulopelvic ligament, it enters the uterus through the uteroovarian ligament.
3. Anastomosis occurs between the uterine and ovarian vessels near the uterine fundus. The anastomosis between the uterine and ovarian vessels provides the placenta with a low-resistance, high-flow circulation.
4. The uterine and ovarian arteries end as spiral arteries that supply the intervillous space. These arteries bathe the terminal villi of the fetal side of the placenta.
B. Human placenta. The human placenta serves many functions in addition to forming a link between the fetus and mother. The growing fetus is dependent on the placenta for respiratory, endocrine, and renal functions, as well as transport of nutrients and wastes. What is especially remarkable is that the placenta is able to perform these functions without significant mixing of maternal and fetal blood. The placenta undergoes changes from early gestation, when the uterine environment is relatively hypoxic, to a term placenta that is highly vascularized. The placenta grows during pregnancy in direct correlation with fetal growth, reaching a diameter of 16 to 19 cm and a weight of 500 g at term.4
1. Macroscopically, the full-term placenta consists of the chorionic plate, which is considered the fetal side of the placenta, and, on the maternal side, the basal plate, which is attached to the maternal endometrium. There are three types of placentas found in mammals: hemochorial, endotheliochorial, and epitheliochorial based on the layers between maternal and fetal blood.4
a. The human placenta is considered to be hemochorial (i.e., maternal blood is in direct contact with the chorionic trophoblast).
b. There is a separation of the maternal basal plate and fetal chorionic plate. It is at the separation of these two plates that causes the maternal spiral arteries to surround the fetal villous trees with blood as the trees enter through the chorionic plate.5
2. Microscopically, placentation begins with implantation of the blastocyst (precursor to the embryo) into the lining of the uterus (i.e., maternal endometrium).
a. Villi, which are the functional units on the fetal side of the placenta, develop during the first trimester. The trophoblast cells invade the maternal endometrium and proliferate with finger-like outgrowths. These outgrowths are termed primary villi.
b. The primary villi continue to branch through angiogenesis and form subsequent generations of villi. The branching of villi creates a dense network of capillaries that reduce the vascular impedance and provide greater surface area for the exchange of nutrients and wastes.6
c. Spiral arteries receive the maternal blood flow from the uterine and ovarian arteries and supply blood to the intervillous space, which contain the terminal villi.6
d. The villous membrane that separates maternal and fetal blood consists of the syncytiotrophoblast, matrix of the villous core, and the endothelium of the fetal capillary. It is through this membrane that gas, nutrient, and drug exchange occur between the fetus and the mother.6
C. Fetal circulation. The fetal circulation begins with the paired umbilical arteries that are branches of the fetal internal iliac arteries. These arteries carry deoxygenated blood from the fetus to the placenta.
1. Umbilical arteries branch into smaller umbilical capillaries that feed the placental villi.
2. After the fetal blood passes through the terminal villus, it leaves the placenta through a single umbilical vein.
3. The umbilical vein enters the fetus and travels through the fetal liver and joins the hepatic vein.
4. The umbilical and hepatic veins empty into the ductus venosus, which enters the right atrium.
5. Oxygenated blood can cross the right atrium through the foramen ovale into the left atrium where it will enter the main fetal circulation.
II. Uteroplacental circulation
A. Placental circulatory development. Successful development of the fetal and maternal circulation requires successful invasion of the fetal trophoblast into the decidua; remodeling of maternal spiral arteries with loss of arterial musculature; and conversion to a high-volume, low-resistance system for maternal blood flow.7
1. Low oxygen tension stimulates placental angiogenesis through activation of hypoxia inducible factor-1α, which activates vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthetase (eNOS). This allows successful trophoblast invasion. Relative hypoxia is required for successful angiogenesis because the presence of reactive oxygen species within the trophoblast results from a higher oxygen environment and is thought to interfere with vascular development.8
2. Remodeling of the spiral arteries occurs during early implantation.
a. Failure of the spiral arteries to remodel is the anatomic defect nearly always seen in women with preeclampsia.
b. Early, higher than normal oxygenation in the intervillous space is thought to be a primary pathogenic factor, leading to reduced blood flow and failure of vascular development.8
CLINICAL PEARL Remodeling of the maternal arteries that supply blood to the intervillous space is essential for normal fetal development. Its failure is the main pathogenic feature of preeclampsia.
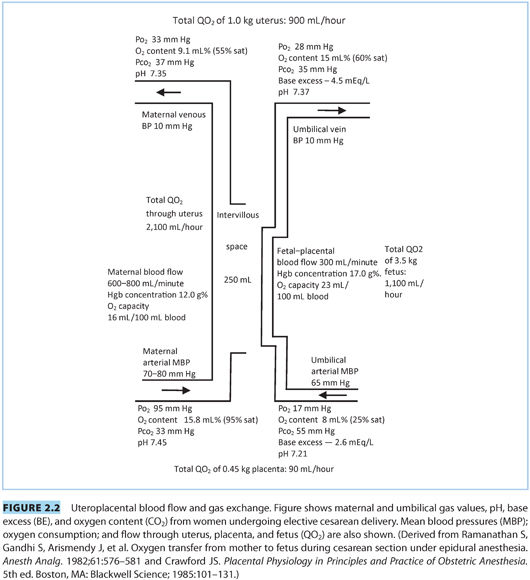
B. Uterine blood flow (see Fig. 2.2). At term, uterine blood flow (UBF) is approximately 700 mL per minute of which approximately 70% to 90% passes through the intervillous space. The rest supplies the metabolic demands of the myometrium.9
1. The uterine arterial bed is thought to be maximally vasodilated at term, with little ability to dilate further because it is not autoregulated.10 During labor, uterine blood flow is intermittently interrupted by uterine contractions and cord compression; this is the most probable mechanism by which fetal compromise occurs during most normal labors. Blood flow is determined by the gradient/resistance relationship:
UBF = uterine artery pressure − uterine venous pressure ÷ uterine vascular resistance
2. A variety of factors can lead to decreases in uterine blood flow.11
a. Regular uterine contractions and abnormal uterine hypertonus due to placental abruption, tachysystole, and excess α-adrenergic agent activity (most often due to excess endogenous maternal catecholamines) are causes for decreased blood flow during labor.
b. Maternal hypotension due to sympathetic block, aortocaval compression, low cardiac output, and relative maternal hypovolemia (as might occur during neuraxial analgesia/anesthesia) as well as hemorrhage and poor patient positioning, can contribute.
c. Acute maternal hypertension (chronic, medication induced [e.g., cocaine]) can lead to placental abruption; chronic hypertension can cause uterine vessel abnormalities over time, chronically reducing blood flow.
d. The administration of exogenous vasoconstrictors (most often α-adrenergic agents) can reduce blood flow; however, their use is indicated when treating hypotension associated with neuraxial analgesia/anesthesia.
e. Local anesthetics can reduce uterine blood flow if injected intravascularly.
f. Uterine tone can increase due to maternal pain relief after neuraxial blockade. This may be related to decreased endogenous sympathetic activity. Maternal epinephrine levels decrease, whereas norepinephrine levels tend to be maintained during neuraxial analgesia, perhaps leading to a relative increase in α-adrenergic activity.
CLINICAL PEARL Increases in uterine tone leading to alteration of fetal heart rate can accompany the onset of neuraxial analgesia. This is probably due to changes in maternal catecholamines.
3. There are few useful mechanisms to increase uterine blood flow.11 Treatment of chronic hypertension with antihypertensives (such as hydralazine) can improve blood flow. Neuraxial analgesia administered during labor is associated with an increase in uterine blood flow, especially in women with preeclampsia. However, these and other measures are not of clinical usefulness.11
4. Measurements of uteroplacental blood flow based on uterine artery flow may not accurately reflect total flow due to ovarian artery contributions.12
a. Intervillous blood flow closely approximates clinically relevant placental flow. In laboratory animals, flow can be measured following the injection of traceable compounds by use of Fick equation calculations. This requires collecting total venous outflow from the uterus, which is impossible in human studies. Injections of trace amounts of xenon 133 and calculation of radioactive decay over the uterus or administering radiolabeled albumin have been used in clinical studies of human intervillous flow.12
b. Doppler ultrasonography of the uterine artery near its take off from the iliac artery at the pelvic brim is the most clinically relevant means of assessing uterine blood flow. The relationship below is used to calculate blood flow:
Q = VRBC × A where VRBC = (Δf/ / fo) × (c / 2 × cos θ)
where Q is total flow, VRBC is the red blood cell velocity, Δf/ is Doppler shift in sound wave frequency, fo is the initial sound wave frequency, c is speed of sound in tissue, and θ is angle of the probe to the axis of the artery. Because the results rely on accurate measurement of θ, indices that can be derived from the Doppler waveform that do not depend on θ can be followed to assess vascular impedance over time (systolic/diastolic ratio, pulsatility index, resistance index).12
C. Umbilical blood flow. Umbilical blood flow is approximately 100 to 120 mL/kg/min at term when measured by Doppler ultrasound (see Fig. 2.2).
1. The fetoplacental circulation has no systemic sympathetic nervous innervation except the most proximal portions of the umbilical vessels. Thus, fetal regulation of blood flow is determined by circulating hormonal effects (primarily catecholamines) and local autoregulatory effects mediated by nitric oxide and acetylcholine metabolism.13
2. Hypoxia induces vasoconstriction in the fetal circulation probably by reducing endothelial released nitric oxide and thus may redistribute blood flow in the fetal circulation much like hypoxic pulmonary vasoconstriction in the lung.14
3. Doppler ultrasound can be used to assess umbilical artery blood flow by measuring red blood cell velocity and using the relationship described above. The pulsatility index, resistive index, and systolic/diastolic ratio can be followed over time as an assessment of fetal artery resistance in obstetric disease states (intrauterine growth restriction, preeclampsia). These measurements can help determine the time for optimal delivery.15
III.Respiratory gas exchange (see Fig. 2.2). Oxygen demand by the fetus is higher than in adults (8 mL 2/min/kg body weight vs. 4 mL/min/kg body weight). The placenta is much less efficient than the lung for gas exchange with only 30% of its surface area available for gas transfer and a thicker membrane across which diffusion occurs. About 20% of uterine arterial blood and 40% of the oxygen provided by the uterine artery are shunted away from gas exchange to serve the metabolic demands of the placenta and uterine tissue; this shunt is stable over a wide range of maternal blood pressures and oxygen saturations.16 The fetal and maternal circulation most likely contact each other in a concurrent fashion, thus near the equilibration of maternal vein and umbilical vein PaO2 and PCO2.17 The respiratory gas values noted in Figure 2.2 may differ from those noted in other texts17 probably because the values noted in Figure 2.2 are from women undergoing elective cesarean delivery versus values published elsewhere from women undergoing labor and vaginal delivery, which will reflect the stress of labor.
CLINICAL PEARL Prolonged labor can be associated with a progressive degradation of the fetal intrauterine environment over time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







