Table 1.2
Local anesthetics with an amide bond
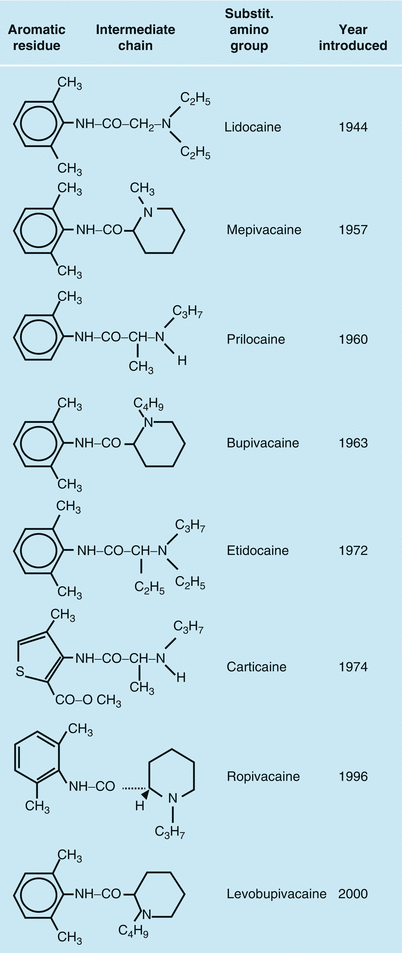
A substituted amino group, the protonization of which determines the ratio of the cationic to the basic form. Only the free base is capable of penetrating lipoprotein membranes. However, to be able to affect the nerve membrane, the local anesthetic must be available as a cation. The type of amino group substitution affects the distribution coefficient, the plasma protein binding, and the intensity and duration of the drug’s action.
Clinical Significance of the Physicochemical Properties
Local anesthetics differ with regard to their molecular weight, their lipid and water solubility, pKa, and protein-binding characteristics. These factors in turn have a substantial influence on the potency of the drug’s local anesthetic effect on the onset of the effect and on its duration (Tables 1.3a and 1.3b).
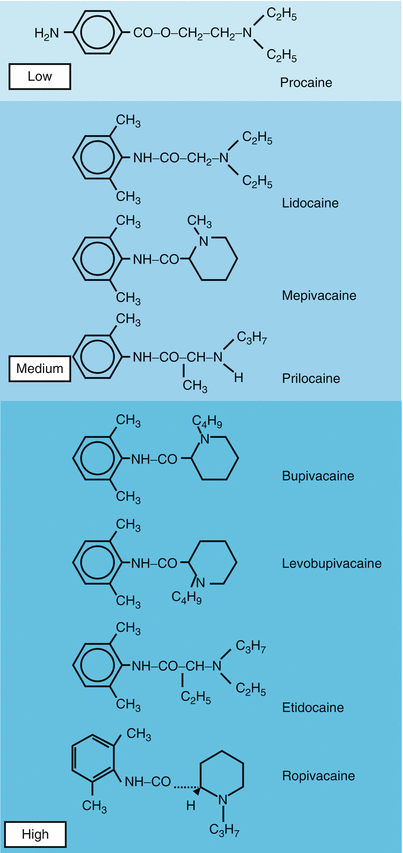
Table 1.3a
A physicochemical and pharmacological parameters
Agent | Molecular weight | pKa (25°) | Distribution coefficient (lipid/water) | Protein binding (%) | Potency in vitro (isolated nerve) |
|---|---|---|---|---|---|
Procaine | 236 | 8.9 | 0.02 | 5.8 | 1 |
Lidocaine | 220 | 7.7 | 2.9 | 64–70 | 4 |
Mepivacaine | 234 | 7.7 | 0.9 | 77–80 | 3–4 |
Prilocaine | 246 | 7.6 | 0.8 | 55 | 3–4 |
Bupivacaine | 288 | 8.1 | 27.5 | 95 | 16 |
Etidocaine | 276 | 7.7 | 141 | 95 | 16 |
Ropivacaine | 274 | 8.1 | 9 | 95 | 16 |
Levobupivacaine | 288 | 8.09 | 27.5 | 97 | 16 |
Table 1.3b
Local anesthetic potency and duration of effect

Local Anesthetic Potency [2]
The combined effect of factors such as protein binding, stereoisomeric structure, and lipophilia determines the potency of a local anesthetic agent. To achieve a blocking effect, the local anesthetic has to diffuse across the cell membrane into the interior of the cell (importance of lipophilia for membrane diffusion) so that, from the cytosol (appropriate hydrophilic properties), it can occupy the sodium channel in its then protonated form (Table 1.4).
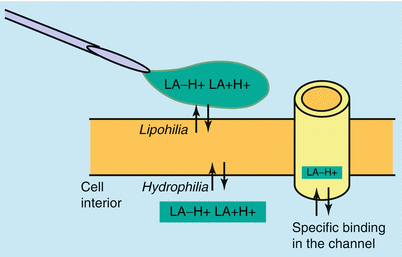
Table 1.4
Chemical requirements of a local anesthetic

A high degree of lipophilia is associated with good membrane permeation, and a high degree of hydrophilia is associated with good solubility in the cytosol. Local anesthetics therefore have to have both of these properties in a favorable ratio.
However, the clinical distinction that is made in local anesthetics between those of mild potency (procaine), medium potency (lidocaine, prilocaine, mepivacaine), and high potency (ropivacaine, bupivacaine, levobupivacaine, etidocaine) does not conform to these correlations in all respects.
The onset of effect in the isolated nerve, at physiological pH, depends on the pKa value of the local anesthetic. The lower this value is, the more local anesthetic base can diffuse toward the membrane receptors, and the shorter the time will be to the onset of the nerve block. Higher concentrations of local anesthetic accelerate onset.
The duration of effect depends on the dosage and concentration of the local anesthetic, its binding to the membrane receptors (protein-binding capacity), and its reabsorption from the tissue into the blood.
Equipotent Concentrations
Medium-duration local anesthetics have more or less the same clinical potency (except perhaps for lidocaine—due to stronger vasodilation, this local anesthetic is resorbed more readily from the site of action, and this can affect the duration and intensity of the block).
Equipotent concentrations of long-acting local anesthetics cannot be demonstrated in the same way, since the three local anesthetics mentioned have completely different block profiles: etidocaine (highest lipophilic capacity) produces a mainly motor block, ropivacaine has a mainly sensory effect, and bupivacaine has both motor and sensory effects. Anesthetic concentrations of bupivacaine and ropivacaine are equipotent (one to one).
Block Profile (Table 1.5)
Table 1.5
Relative block profile of long-acting local anesthetics
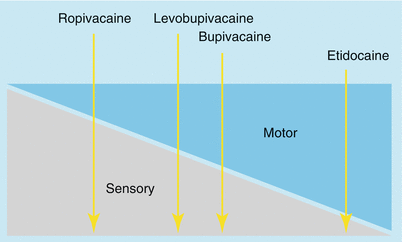
The block profile shows the relation between sensory and motor block. Physicochemical properties determine the block profile. At high anesthetic concentrations—so far as these are toxicologically permissible—the excess quantity of the agent can also block fibers not primarily affected (motor or sensory fibers). On the other hand, the block profile is not altered by low concentrations. A reduced motor block is obtained at the cost of reduced analgesic quality, and this is why opioid supplementation is usually necessary with dilute concentrations of local anesthetic.
Incompatibility
Local anesthetics can precipitate after dilution with alkaline solutions and should therefore not be diluted with or injected simultaneously with sodium bicarbonate.
Side Effects and Systemic Effects (Tables 1.6 and 1.7)
Table 1.6
Toxicity of clinical dosages of local anesthetics
Local anesthetic | Central nervous system | Heart |
|---|---|---|
Lidocaine | ++ | + |
Mepivacaine | ++ | + |
Prilocaine | + | +/− |
Bupivacaine | +++ | ++++++a |
Levobupivacaine | ++ | ++++ |
Ropivacaine | ++(+) | +++ |
Table 1.7
Symptoms of intoxication due to local anesthetics
Central nervous system | Cardiovascular system |
|---|---|
Stimulation phase, mild intoxication | |
Tingling of lips, tongue paresthesias, perioral numbness, ringing in the ears, metallic taste, anxiety, restlessness, trembling, muscle twitching, vomiting | Cardiac palpitation, hypertonia, tachycardia, tachypnea, dry mouth |
Stimulation phase, moderately severe intoxication | |
Excitation phase, moderate toxicity Speech disturbance, dazed state, sleepiness, confusion, tremor, choreoid movements, tonic-clonic cramp, mydriasis, vomiting, polypnea | Tachycardia, arrhythmia, cyanosis and pallor, nausea and vomiting |
Paralytic phase, severe toxicity | |
Stupor, coma, irregular breathing, respiratory arrest, flaccidity, vomiting with aspiration, sphincter paralysis, death | Severe cyanosis, bradycardia, drop in blood pressure, primary heart failure, ventricular fibrillation, hyposystole, asystole |
When assessing the safety and tolerability of a local anesthetic, not only its central nervous system and cardiovascular effects need to be taken into account, but also its allergenic potential and toxic degradation products that may form as it is metabolized.
Systemic Effects
Adverse systemic effects of local anesthetics can occur when their plasma concentration is high enough to affect organs with membranes that can be irritated.
Toxic plasma levels can be reached as a result of:
Inadvertent intravascular or intrathecal/epidural injection
Overdosing, particularly in areas with good perfusion and correspondingly high resorption
Failure to adjust the dosages (mg/kg body weight), particularly in patients with hepatic or renal disease
The severity of intoxication depends on the absolute plasma level, as well as on the strength of the local anesthetic’s effect. While anesthetic dosages of short-acting local anesthetics (prilocaine, mepivacaine, lidocaine) can trigger clear CNS symptoms in a range extending to generalized cramp, cardiotoxic reactions are also possible with long-acting local anesthetics. In particular, cases of cardiac arrest have been reported with bupivacaine with comparatively small intravascular injections (50 mg; not treatable in half of the cases).
Cardiac symptoms and cardiac arrest can also occur with ropivacaine after inadvertent intravascular injections. However, these can be treated effectively and only occur at higher dosages. The following sequence of increasing systemic toxicity applies to the most frequently used local anesthetics: procaine < prilocaine < mepivacaine < lidocaine < ropivacaine < levobupivacaine < bupivacaine.
CNS toxicity: Central reactions predominate in terms of frequency and clinical significance. The symptoms of these are listed in Table 1.7 in order of severity and toxicity. For speedy and appropriate treatment, it is important to observe and react immediately when even the preconvulsive signs of CNS intoxication are seen—particularly numbness of the tongue and perioral region.
Cardiovascular toxicity: Toxic effects on the cardiovascular system usually occur after the administration of very high doses. They are seen in the form of conduction disturbances in the autonomic cardiac and vascular nerve fibers, depression of cardiac function, and peripheral vasodilation (Tables 1.6 and 1.7).
Local Anesthetic Systemic Toxicity (LAST) [3–7]
Diagnosing (Table 1.7 )
Classic descriptions of LAST depict a progression of subjective symptoms of CNS excitement (agitation, auditory changes, metallic taste, or abrupt onset of psychiatric symptoms) followed by seizures or CNS depression (drowsiness, coma, or respiratory arrest). Near the end of this continuum, initial signs of cardiac toxicity (hypertension, tachycardia, or ventricular arrhythmias) are supplanted by cardiac depression (bradycardia, conduction block, asystole, decreased contractility) [3]. However, there is substantial variation of this classic description, including the following:
Simultaneous presentation of CNS and cardiac toxicity
Cardiac toxicity without prodromal signs and symptoms of CNS toxicity
Thus, the practitioner must be vigilant for atypical or unexpected presentation of LAST. The timing of LAST presentation is variable: Immediate (<60 s) presentation suggests intravascular injection of LA with direct access to the brain, while presentation that is delayed 1–5 min suggests intermittent or partial intravascular injection, delayed circulation time, or delayed tissue absorption. Because LAST can present >15 min after injection, patients who receive potentially toxic doses of LA should be closely monitored for at least 30 min after injection.
Caution
The onset of LAST is usually very rapid, following a single LA injection by 50 s or less in half of the cases and occurring before 5 min in ¾ of the cases [3]. The most important first step in improving patient outcome is to have a low threshold for considering the diagnosis (atypical presentation was reported in approximately 40 % of published cases of LAST) [6].
Prevention [6]
Prevention is the most important measure in reducing the frequency and severity of LAST. No single intervention has been identified that can reliably eliminate risk. Central to prevention is limiting the opportunity for intravascular injection or tissue uptake to local anesthetic, which is best accomplished by early detection of intravascular needle or catheter placement.
Local anesthetic dose reduction may be particularly important for those patients thought to be at greater risk of LAST.
Risk Reduction
Local anesthetic blood levels are influenced by the site of injection, and those factors that can increase the likelihood of LAST include:
1.
Those patients at extremes of age (<4 months or >70 years).
2.
Heart failure, history of ischemic heart disease, and cardiac conduction abnormalities.
3.
Metabolic (e.g., mitochondrial) disease.
4.
Liver disease.
5.
Low plasma protein concentration.
6.
Metabolic or respiratory acidosis.
7.
Medications that inhibit sodium channels.
Neither body weight nor body mass index correlates with local anesthetic plasma levels after a specific dose in adults; the correlation is more accurate in children.
Caution
1.
Use incremental injection of local anesthetics before and during the injection (after each 4–5 mL—aspiration should be carried out repeatedly, pausing 15–30 s between each injection) observing for signs and querying frequently for symptoms of toxicity between each injection recognizing that there is ~ 2 % false-negative rate for this diagnostic intervention [5].
2.
Maintain verbal contact with the patient.
3.
Monitor the patient during and after completing the injection, as clinical toxicity can be delayed up to 30 min (or longer after tumescent procedures).
Intravascular Marker
When injecting potentially toxic doses of local anesthetic, use of an intravascular marker is recommended [5]. Although imperfect, intravascular test dosing remains the most reliable marker of intravascular injection. Of the various options described, only fentanyl and epinephrine meet suggested standards for reliability and applicability [4, 6].
Intravascular injection of epinephrine 10–15 μg/mL in adults produces a ≥10-beat HR increase or a ≥15-mmHg SBP increase in the absence of ß-blockade, active labor, advanced age, or general/neuraxial anesthesia. Intravascular injection of epinephrine 0.5 μg/kg in children produces a ≥15-mmHg increase in SBP [8]. Nevertheless, epinephrine test doses are unreliable in the elderly or in patients who are sedated, taking ß-blockers, or anesthetized with general or neuraxial anesthesia. Fentanyl 100 μg produces sedation if injected intravenously in laboring patients [5].
Ultrasound
Ultrasound guidance may reduce the frequency of intravascular injection, but actual reduction of LAST remains unproven in humans.
Recommendations for Treatment of LAST (Table 1.8) [3, 6, 7]
Table 1.8
Recommendation for treatment of LAST
(a) |
1. Be prepared. Establish a plan and checklist for managing |
2. If signs and symptoms of LAST occur, prompt and effective airway management (ventilate with 100 % oxygen) is crucial to preventing hypoxia and acidosis |
▼ |
3. Immediate treatment of convulsions within 15–30 s of their onset especially correcting hypoxia and acidosis is not associated with cardiac catastrophe [7] |
4. Get help |
(b) |
If seizures occur |
1. Benzodiazepines are preferred |
2. If benzodiazepines are not readily available, |
▼ |
Small doses of propofol (0.5–1.5 mg/kg) or thiopental (1–2 mg/kg) are acceptable
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





