Etiology
Frequency (%)
Duodenal ulcer
33.2
Gastric ulcer
16.9
Varices
11.5
Gastric erosions
10.4
Neoplasms
5.2
Esophagitis
4.3
Mallory–Weiss lesions
3.2
Anastomotic ulcer
3.0
Vascular malformations
2.8
Rare causes, including transpapillary bleeding
2.4
Undetermined
5.0
Peptic Ulcer Disease
Peptic ulcer disease remains the most common etiology of UGIB. Duodenal ulcers are the most common cause of UGIB both historically and currently, accounting for 20–30% [2, 4, 5]. Unfortunately, most studies include all duodenal ulcers, gastric ulcers, and often gastritis under peptic ulcer disease, despite the evidence that several of these conditions have different etiologies and potentially different surgical management. The four major causes of gastric and duodenal inflammation, ulceration, and bleeding are overproduction of acid, H. pylori infection, nonsteroidal anti-inflammatory drug (NSAID)-induced loss of mucosal defense, and stress-induced loss of mucosal defense. Gastric acid and pepsin produced in the stomach, both important for breakdown of food, are cytotoxic to the cells of the stomach and small intestine. The cells lining these organs are protected from these agents by a thick layer of mucus. When acid production and mucosal defense mechanisms are in balance, acid and pepsin can be safely secreted without harm to the tissue. If one of these components is altered, breakdown of the tissue with resultant inflammation, ulceration and bleeding may occur. The least common cause is true acid-overproduction, as seen in Zollinger–Ellison syndrome. The autoregulation of acid secretion is disrupted and overwhelms mucosal defense mechanisms.
All other causes of inflammation, ulceration, and bleeding are essentially caused by disruptions in mucosal defense. H. pylori, a motile bacterium which lives in the mucus layer of the stomach, secretes several different cytotoxins that weaken the mucus barrier protecting the underlying cells as well as damage the cells directly. Increased permeability of the mucus layer allows for direct damage by gastric acid and pepsin; underlying cell damage promotes an inflammatory response and decreases mucus production. It is estimated that 70% of gastric ulcers and 95% of duodenal ulcers are associated with H. pylori infection [6].
A second mechanism by which mucosal defenses are broken down is NSAID use. NSAIDs block cyclooxygenase (COX) activity. COX exists in two forms in the body, COX1 and COX2, and most NSAIDs on the market today inhibit both enzymes to some degree. COX1 is a constitutive enzyme in the gastric mucosa that converts arachidonic acid into prostaglandins. Prostaglandins play a cytoprotective role in the stomach by stimulating mucin, bicarbonate, and phospholipid secretion by epithelial cells. They stimulate local mucosal blood flow and promote epithelial cell division and migration, all important components of mucosal repair. NSAIDs also appear to interfere with several other mechanisms important to mucosal defense such as inducible nitric oxide activity but these remain less well defined at this time. Aspirin irreversibly binds to COX1 and the gastric mucosa requires 5–8 days after the last dose of aspirin to regain levels of COX1 adequate to perform its cytoprotective role. The transient inhibition of COX1 by other NSAIDs such as ibuprofen and naproxyn allows for a faster recovery of cytoprotection, but consistent use of these agents causes as much damage as aspirin [7]. The combination of H. pylori infection with NSAID use has a synergistic, additive effect on peptic ulcer disease. Both factors increase ulcer formation independently. H. pylori infection increases ulcer incidence fourfold, whereas NSAID use increases ulcer incidence threefold. The presence of both increases the risk of ulcers 17-fold over patients who were H. pylori negative and non-NSAID users. More importantly, NSAID use increases risk of UGIB by fivefold over nonusers. H. pylori infection and NSAID use together increased the risk of bleeding 20-fold over noninfected, nonusers [8].
Gastric ulcers have been categorized by their location in the stomach as well as by their association with acid secretion. The original classification scheme subdivided gastric ulcers by their location in the stomach. Type I ulcers are located on the lesser curvature of the stomach, while Type II ulcers are located on the lesser curvature and are accompanied by a duodenal ulcer. Type III ulcers are located in the prepyloric region. Type IV ulcers are located in the cardia near the GE junction. Type V ulcers are diffuse. Several studies done during the era prior to the discovery of H. pylori correlated gastric acid secretion levels with the locations of these ulcers [9, 10]. The evidence suggested Types II and III were associated with high gastric acid secretion and Types I, IV, and V were not. Unfortunately, this data has not been updated since the discovery of the role of H. pylori in the etiology of peptic ulcer disease. Infection with H. pylori can cause hypergastrinemia and increase gastric acid secretion [11]. Moreover, there is data demonstrating no correlation between the location of a gastric ulcer and basal acid output [12]. Duodenal ulcers seem to be most reliably associated with high acid secretion; they are also overwhelmingly associated with H. pylori infection. Further work needs to be done in this area and the historical surgical classification of ulcers based on high acid secretion status without consideration the H. pylori factor needs to be reevaluated.
Esophageal and Gastric Varices
Variceal bleeding accounts for 4–30% of all UGIBs depending upon the study population. More importantly, it accounts for 50–60% of UGIBs in cirrhotic patients. In a retrospective study of 403 cirrhotic patients with bleeding varices, 36% were diagnosed with cirrhosis during their first admission for an acute UGIB [13]. These data bring up two important points. First, it is important to assess any patient with an UGIB for signs and symptoms of cirrhosis and pursue this diagnosis with an ultrasound or computed tomography (CT) scan if necessary, as the patient may not have been diagnosed with the disease at the time of their first UGIB. Second, although the slight majority of cirrhotics with UGIBs will have variceal bleeding, a significant number will have a non-variceal source of hemorrhage. This distinction is important for the treatment options available to the patient and treating physician.
Portal Hypertensive Gastropathy
Portal hypertensive gastropathy (PHG) is a poorly understood phenomenon occurring in patients with portal hypertension, usually secondary to cirrhosis, in which alterations in the gastric mucosa results in macroscopic changes in the mucosa as well as mucosal and submucosal vascular ectasia without histological evidence of inflammation. This has been reported to cause acute and chronic UGIB in patients with cirrhosis or portal hypertension. The incidence of PHG as a cause of UGIB is difficult to estimate because the patients who present with this phenomenon may be misdiagnosed as having variceal bleeding, acute gastritis, or angiodysplastic lesions. These lesions appear to be caused by local changes in vascular tone, nitric oxide, and various cytokines. There is debate in the literature about the relationship between the degree of portal hypertension as measured by the hepatic venous pressure gradient and the presence or severity of PHG. The relationship between the severity of PHG and the severity of hepatic dysfunction, portal hypertension, and causes of hepatic dysfunction remain unclear [14].
Mallory–Weiss Tear
Historically known as Mallory–Weiss syndrome, this cause of UGIB was originally associated with a triad of signs and symptoms: vomiting, hematemesis, and alcohol abuse. The lesions found at endoscopy were acute linear mucosal tears at or around the gastroesophageal junction, often with brisk associated arterial bleeding. These tears are thought to be caused by rapid forceful dilation of the GE junction. It is now understood that this process can result from many different activities, including childbirth, esophageal intubation, seizures, blunt abdominal trauma, cardiopulmonary resuscitation (CPR), and many different causes of sustained retching or vomiting, including hyperemesis gravidarum, alcohol intoxication, and chemotherapeutic treatments. On endoscopy, the most common appearance is a single linear tear along the lesser curvature of the stomach at the GE junction. Less frequently, there is more than one tear and if present, the other sites are along the greater curvature, posteriorly, and very rarely anteriorly. Up to 90% of Mallory–Weiss lesions stop bleeding spontaneously with resuscitation, acid suppression, and antiemetics [15].
Neoplasm
Benign tumors of the esophagus, stomach, and duodenum will cause bleeding as their initial symptom in approximately 10% of patients. This is usually the result of ulceration of the mucosa overlying a submucosal mass. Malignant tumors of this area will also infrequently bleed although it is not a common presenting symptom. Malignancies in this area include esophageal cancer, gastric adenocarcinoma, gastrointestinal stromal tumors (GISTs), and duodenal adenocarcinomas. Massive UGIB secondary to neoplastic disease is uncommon.
Dieulafoy Lesions
Dieulafoy lesions, first accurately identified in 1898, are a rare cause of UGIB. They account for between 3 and 5% of UGIBs, although they are likely underreported and misidentified as arteriovenous malformations and angiodysplastic lesions. Pathologically, a Dieulafoy lesion results when an artery travelling through the wall of the gastrointestinal tract fails to narrow appropriately as it reaches the outer limits of its territory. This has also been described as a “caliber-persistent artery” in that it is a histologically normal vessel other than its constant diameter of 1–3 mm. Macroscopically these vessels run a tortuous course in the submucosa. Bleeding occurs when the mucosa overlying the vessel breaks down and the wall of the vessel is injured or eroded. These lesions are not evenly distributed throughout the gastrointestinal tract. 71% are found in the stomach, 15% in the duodenum, and 8% in the esophagus. The remaining 6% are relatively evenly distributed in the rest of the small bowel, colon and rectum. Bleeding Dieulafoy lesions are more common in elderly patients already hospitalized. 90% of patients with lesions will have comorbidities and 40–50% will be taking NSAIDs, aspirin, or warfarin. These data suggest stress or impairment of mucosal defense mechanisms leading to mucosal breakdown and vessel erosion may be the proximal cause of bleeding in these lesions but this link remains elusive. Bleeding from a Dieulafoy lesion can range from intermittent to massive and correspondingly, presentation can range from iron-deficiency anemia to combined hematemesis and melena [16, 17].
Aortoenteric Fistula
A primary fistulous connection between the upper GI tract and aorta is very rare. If present, it is associated with an atherosclerotic aortic aneurysm in 85% of patients. Much less likely causes include foreign body ingestion or an infectious, inflammatory or neoplastic process. On the other hand, aortoenteric fistulae after aortic reconstruction are far more common. The third portion of the duodenum often directly overlies the repair and is involved in 80% of all aortoenteric fistulae [18, 19]. Less common sites of secondary fistulization include the esophagus and stomach. The classic presentation of an aortoenteric fistula is abdominal pain, a pulsatile mass, and a spontaneously resolving “herald” GIB, followed eventually by massive bleeding, exsanguination, and death.
Transpapillary Hemorrhage
A less common but important group includes those pathologies that result in hemobilia, or transpapillary bleeding. The most common cause of transpapillary bleeding is iatrogenic injury from hepatic or biliary procedures done percutaneously or endoscopically. Others include trauma, malignancy, hepatic artery aneurysms, arteriovenous malformations, and hemosuccus pancreaticus [4, 20]. Hemosuccus pancreaticus occurs when a pancreatic pseudocyst or other pancreatic inflammatory process erodes the surrounding tissue and forms a communication between the pancreatic duct and a peripancreatic artery or the splenic artery. These patients usually present with abdominal or back pain and hematochezia. Although rare, it is most common in patients with chronic pancreatitis. On endoscopy, the bleeding will appear to be coming from the ampulla of Vater [4, 21].
Clinical Presentation and Initial Management
Patients with an UGIB may present with a range of symptoms depending upon the rapidity and severity of the bleeding. Symptoms include hematemesis, hematochezia, melena, occult fecal blood, anemia and fatigue. Hemodynamic instability may or may not accompany the symptoms.
Resuscitation
Management of an UGIB should begin before a diagnosis is made. Although many UGIBs will stop spontaneously, the clinicians treating a patient with an UGIB should assume it will not. All patients with an UGIB should be managed initially by following the fundamentals of resuscitation of shock. The patient’s capacity to maintain a safe airway should be established and if in doubt, the patient should be intubated. The patient’s cardiovascular status and the presence or absence of shock should be rapidly investigated and treated. If the patient has any signs of hemodynamic instability or visible evidence of large volume blood loss, the patient should have large-bore vascular access placed and the blood bank should be alerted for possible unmatched blood needs and a type and crossmatch for packed red blood cells. The physical exam is not as helpful in UGIB as it can be in other disease processes. Signs of shock, stigmata of liver disease, presence of peripheral vascular disease, and evidence of previous surgery can usually be investigated. If gross blood or melena is not obvious, a digital rectal exam and fecal occult blood test should not be overlooked. Bloodwork should include a complete blood count, a coagulation panel, a comprehensive metabolic panel and a blood type and screen, at minimum. If there is any history that suggests the patient will have abnormal coagulation studies, dysfunctional, or absent platelets, fresh frozen plasma and platelets should be made available as well [21].
A complete blood count is useful for ruling out blood dyscrasias, and the presence or absence of thrombocytopenia. The hemoglobin level is useful; however, a trend in hemoglobin levels over time is usually more helpful depending on the clinical situation. The metabolic panel will be most helpful in assessing the patient’s current level of renal or hepatic dysfunction, if any. Coagulation panels are useful in assessing the current level of coagulation impairment and should be correlated to medication history. If a patient is coagulopathic and not taking an anticoagulant, malnutrition, DIC, and underlying liver disease should be urgently considered and investigated. Consideration should be given to ordering platelet function assays in patients taking platelet-altering medications. There is some data to recommend platelet transfusions to normalize function assays in patients taking platelet inhibitors who are having a significant bleeding problem [22]. This has not been studied in the setting of UGIB but deserves consideration in the patient with a massive or hemodynamically significant bleed.
History
Concurrent to these events, as much history as possible should be elicited from the patient, the family, and any other physicians involved with the patient. In addition to the events surrounding the UGIB, attention should be paid to any previous history of peptic ulcer disease or other forms of GIBs. Also, any history of liver disease, renal disease, malignancy, and cardiovascular disorders will be important as each of these has an implication for the etiology and management of the UGIB. A medication history is vital and must include not only prescription medications, but also over-the-counter medications. Patients frequently have more difficulty identifying the type and quantity of over-the-counter drugs they are taking and often do not know the importance of their NSAID use, for example, in their diagnosis and management. The past surgical history is vital to obtain. The success of any possible intervention in these patients in particular relies on a clear understanding the pre-procedural anatomy. Any prior surgery of the foregut, hepatopancreaticobiliary system, aorta, peripheral vasculature, and other abdominal organs will alter the diagnostic and technical approach of the endoscopist, interventional radiologist, and acute care surgeon.
Nasogastric Intubation and Aspiration
The diagnostic pathway in an UGIB frequently overlaps with the treatment pathway but the first step is identifying the location of the blood loss as proximal or distal to the ligament of Trietz. Every step taken to localize and characterize the source of the bleed improves the chance of successful treatment. A common tool to differentiate an UGIB from lower gastrointestinal bleeding (LGIB) is placement of a nasogastric tube and evaluation of the aspirate. The conventional wisdom suggests a nasogastric aspirate which returns bilious, but not bloody, fluid should prompt a workup for a LGIB. The logic underlying this step is that an aspirate containing bile reflects the fluid composition in the duodenum as well as the stomach; thus, bilious non-bloody fluid should rule out a source proximal to the ligament of Trietz. Unfortunately, this intuitive step has little evidence to support its use. Palamidessi et al. reviewed the literature for studies correlating findings from a nasogastric aspirate with an esophagogastroduodenoscopy (EGD) in patients with melena or hematochezia without hematemesis. They found only three studies that fit their criteria. Among these the sensitivity and specificity of a positive NGT aspirate ranged from 42 to 84% and 54 to 91%, respectively, with the highest values in a cohort of inpatients being treated for a myocardial infarction when they began to have symptoms [23]. They conclude that a conversation with the consulting gastroenterologist concerning the value of an NGT may be worthwhile. If an endoscopist has assessed the patient and their plan to perform upper or lower endoscopy will not be affected by the results of an NGT aspirate, then foregoing this step may be reasonable. Otherwise, an NGT aspirate may still be considered helpful and warranted.
Diagnosis and Management
Diagnostic and Therapeutic Endoscopy
After the initial resuscitation is underway, the source and etiology of the UGIB must be localized. The best initial procedure is usually endoscopy. EGD has proven to be the most important initial procedure. This modality in skilled hands will also offer several therapeutic options. Localization of the source of the bleed is critical. The success of all endoscopic, angiographic, and surgical interventions depends upon localization of the bleeding source. At the minimum, and with the worst conditions, an EGD can differentiate an UGIB from a LGIB. Should the bleeding fail to stop spontaneously or be controlled endoscopically, narrowing down the source vessels or tissues as much as possible makes the possibility of a successful angiographic or surgical intervention with the least amount of morbidity much more likely.
Therapeutic options for the endoscopist have expanded considerably in the last two decades. The techniques used will depend upon the skill and comfort of the endoscopist and the site and etiology of the bleeding. Options include epinephrine or sclerosant injection, thermal coagulation, hemostatic clip application, banding, or a combination of these techniques. Multiple trials have demonstrated that all of these modalities have similar efficacy in arresting bleeding, preventing rebleeding, and reducing the need for urgent surgical intervention. Success after initial treatment can be as high as 98% [4, 24]. Additionally, biopsies can provide useful information and in some institutions, H. pylori testing from biopsied tissue can be done with results much faster than serology or other types of testing. Neoplastic causes of bleeding can also be established by biopsy and can play an important role in treatment strategies. Furthermore, in the case of transpapillary bleeding, endoscopic retrograde cholangiopancreatography (ERCP) can be helpful for both localization and treatment. Recurrent bleeding after endoscopic control is infrequent, occurring in <10% of patients in centers with strong endoscopy departments. Repeat endoscopy for bleeding after initial endoscopic control has been compared to surgical intervention and has been demonstrated to achieve long-term hemostasis in a majority of patients while avoiding the complications of surgery [24]. Repeat endoscopy is widely regarded as the appropriate response to evidence of rebleeding after initial endoscopic control [21].
CT Scan
Several studies have shown a role for high resolution multidetector CT (MDCT) scan with digital subtraction angiography in localizing GI bleeding, including UGIBs. The “pooled” sensitivity and specificity of MDCT for GIB are 86% and 95%, respectively [25]. In swine and in vitro models, high quality MDCT imaging has been shown to localize bleeding as slow as 0.35–0.5 ml/min [26]. MDCT is currently considered “usually appropriate” by the American College of Radiology as the next diagnostic step following a negative or inconclusive endoscopy [4]. MDCT may be the best next step in a patient in whom an aortoenteric fistula is suspected or in a postsurgical patient in whom the anatomy is unclear. Additionally, if complications of neoplastic disease are high on the differential diagnosis, a CT scan may be highly valuable in assessing the extent of disease and these data could dramatically change further diagnostic and therapeutic plans. Unfortunately, at this time MDCT offers few possible concurrent therapeutic maneuvers and remains a solely diagnostic modality.
Tagged Red Cell Scan
Technetium-99 m-labeled erythrocyte scans, commonly known as tagged red cell scans, are useful because they can detect bleeding as slow as 0.05–0.1 ml/min. The major drawback to this option is its limited value in localizing the bleeding site. In the case of a LGIB, the relatively immobility of the colon can offer adequate localization for the next therapeutic endeavor. If the source of bleeding is in freely mobile viscera such as the majority of the small bowel, this information is of limited value for any intervention. Surprisingly, given the relatively predictable placement of the stomach and duodenum, studies have shown that tagged red cell scan localization of bleeding lesions in these areas to be frequently inaccurate [27, 28]. Thus, for the UGIB, tagged red cell imaging is rarely appropriate.
Diagnostic and Interventional Angiography
With the exception of the discovery of the role of and treatment for H. pylori in peptic ulcer disease, nothing has revolutionized the treatment of GI bleeding as much as advances in interventional radiology in the last two decades. Initially, angiography was available as a purely diagnostic tool and still plays an important role in that respect. Diagnostic angiography can detect bleeding at rates as low as 0.5 ml/min. Although it is generally not as helpful as endoscopy in characterizing the cause of bleeding, angiography can provide anatomic or structural information about lesions which are bleeding at the time of angiography or those that appear to bleed intermittently. Selective visceral angiography is most helpful if the source is arterial; detection of venous bleeding during the venous phase of the imaging is less reliable. Transcatheter arterial embolization (TAE) of UGIB source vessels involves selective visceral angiography and localization of the bleed. This can be expedited by visualization of previously placed endoscopic clips. Thus, hemostatic clip placement at the time of endoscopy is ultimately useful even when the clip placement is not technically successful [29]. Once the site of hemorrhage has been identified, feeding arteries can be embolized with coils, polyvinyl alcohol particles, gelfoam, iso-butyl-2-cyanoacrylate, ethibloc, or a combination of these products. Most studies found no difference in outcomes based on the type of agent used, although no randomized trials have been done to confirm this observation [4, 30]. If the lesion has been at minimum localized to a region of the stomach or duodenum by endoscopy, but cannot be visualized by angiography, “blind” embolization of the vessels feeding this area can be done successfully.
Multiple studies have demonstrated the benefits of TAE in UGIB when endoscopic attempts are not successful. TAE procedural and early clinical success rates (absence of early rebleeding), for bleeding after failure of endoscopic control range from 75 to 100% and 72 to 78%, respectfully in studies published from 2000 onward [20, 31, 32]. This success is highlighted by the fact that many of these studies were done in patients considered too high risk for surgery. The rebleeding rate after TAE in recent studies ranges from 7 to 30%. The factors associated with the risk of rebleeding after TAE have been extensively studied and in multivariate analysis, the presence of coagulopathy or multiple organ failure are consistently associated with clinical failure or rebleeding [20]. The drawbacks of TAE as a treatment option include the requirement for a skilled interventionalist team with 24-h availability, possible inability to cannulate the access vessel secondary to narrow or tortuous vasculature, and inability to localize the bleeding lesion in areas where blind embolization is not an option. Procedural drawbacks include the need for contrast injection and its potential risk of contrast induced renal insufficiency, groin hematomas and pseudoaneurysms, transient hepatic or pancreatic ischemia after embolization, and late duodenal stenosis secondary to embolic ischemia.
There have been no randomized controlled trials comparing TAE to surgery for UGIBs that have failed endoscopic treatment. Several groups have retrospectively compared the two modalities. There are numerous methodological differences amongst these studies; however, most found that patients who underwent embolization rather than surgery were consistently older, had more comorbidities including coronary artery disease, and were more frequently on anticoagulation therapy at the time of the UGIB. Despite this, most showed no significant difference between surgery and TAE in terms of the incidence of recurrent bleeding (23–43%), nor the need for additional surgery [33–36]. This data strongly supports the argument to proceed with angiography and TAE if endoscopic management fails. In cases of transpapillary bleeding, TAE has had excellent results. In studies of TAE in transpapillary bleeding published since 1999, the technical and clinical success rates of TAE have been 88–100% and 75–100%, respectively. The rebleeding rates in these studies ranged from 0 to 21% with major complication rates of 0–3% [20].
The therapeutic options now available for interventional radiologists are various and are not confined to the angiographic approach. They include transjugular intrahepatic portosystemic shunts (TIPS) as well. TIPS insertion for variceal bleeding unresponsive to endoscopic management has excellent outcomes in cessation of bleeding. If combined with variceal embolization, the rate of rebleeding is reduced further. It is equally efficacious as surgical porto-systemic shunts in cirrhotics. Shunts of either type are associated with an increased risk or exacerbation of hepatic encephalopathy. The newer TIPS stents are associated with a secondary patency rate of 90% or higher; additionally, TIPS can be exchanged percutaneously for occlusion and downsized for medically refractory encephalopathy [4].
Surgery
Historically, surgical intervention was the definitive treatment of UGIBs. Fifty years ago, there were neither endoscopic nor angiographic options; medical treatment of H. pylori was unknown. The etiology of UGIB was different. Peptic ulcer disease was more common and occurred in younger patients. Fewer UGIB patients were elderly, on chronic immunosuppression or anticoagulation; none had freshly placed coronary artery stents. Critical care-related illnesses were infrequent as critical care was in its infancy. Transplants and TIPS procedures were not available for patients with end stage liver disease, nor was dialysis available for patients with end stage renal disease. The surgical therapy of UGIB evolved in a patient population and health care system very different from today. The procedures were developed with few goals in mind: cessation of bleeding and acid suppression for peptic ulcer disease and portosystemic shunts for complications of cirrhosis. The morbidity of these procedures was acceptable as there were seldom any alternatives to surgical therapy. Today, the patient population has changed, the etiologies of UGIBs have changed, and many nonsurgical therapies are available. However, surgery is still required in some cases and the procedures available to the surgeon need to be understood. Some acute care surgeons may work in austere environments in which other modalities are not available locally or regionally. Early surgical intervention is lifesaving if less invasive methods of hemostasis are not options. Once an operation has been selected, it needs to be performed well and efficiently. Fewer and fewer acute care surgeons have seen or done any of the procedures used for UGIB in training.
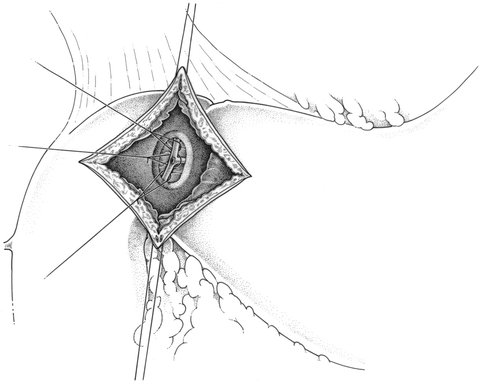
Duodenotomy and Ligation of the Bleeding Vessel
This approach is most frequently used for a bleeding duodenal ulcer on the posterior wall of the lumen. An upper midline incision is made. The duodenum is mobilized and palpated for the location of the ulcer. An anterior longitudinal duodenotomy is made at the level of the posterior ulcer. The bleeding source is usually an erosion into the lumen of the gastroduodenal artery. Nonabsorbable sutures are used to ligate the vessel proximal and distal to the bleeding lumen. A third “U-stitch” is placed medial to the bleeding area to control inflow from the tranverse pancreatic artery (see Fig. 19.1). All of these sutures must be placed with care and precision in order to avoid any injury to the adjacent common bile duct. Once hemostasis has been achieved, the duodenotomy is closed transversely, most often with the Heineke-Mikulicz pyloroplasty, to avoid any postoperative obstructive symptoms [37, 38].