CHAPTER 64 UPPER EXTREMITY VASCULAR TRAUMA
Trauma to the upper extremities can have devastating results. The experience of wartime has taught many lessons about etiology, diagnosis, and treatment of upper extremity injury. Understanding the lessons learned from history provides the basis for treatment today.
Hemorrhage from injured blood vessels has been a well-known consequence of injury for several millennia. The diagnosis was made even in ancient times, but the treatment options were limited. Styptics were prepared from vegetable or mineral material and applied to bleeding vessels. Archigenes first advocated amputation of gangrenous extremities above the line of demarcation with linen ligatures placed on the vessels in the first century ACE.1 Celsus records the first account of vessel ligation to establish hemostasis in 25 ACE1. These ancient recommendations were lost in later centuries, and mass cautery became the standard practice for extremity hemorrhage control until the late 1400s. Ambroise Pare reestablished the technique of amputation above the line of demarcation with linen ligatures of the injured vessels in 1552.1 The tourniquet was introduced in 1674 by Morel. Direct vessel ligation was the treatment of choice by the 19th century, but the results were often disappointing. There was a 100% mortality associated with ligation of the aorta in 10 patients, a 77% mortality with ligation of the common iliac artery in 68 patients, and in 31 patients a 40% mortality when the femoral artery was ligated.2
The first known successful repair of an injured artery was performed in 1759 by Hallowell.3 In 1889, Jassinowsky performed arterial reconstruction in animals and proved that interrupted silk sutures could successfully repair the carotid artery.4 Murphy in Chicago was the first to successfully perform an end-to-end anastomosis on a femoral artery in 1897. He had firm beliefs that successful repair in vascular trauma required complete asepsis, atraumatic technique, temporary clamping of the vessel, accurate approximation, and meticulous hemostasis and cleansing of the wound.2,3 Carrel and Guthrie described the basic techniques still used today for end-to-end anastomosis and lateral suture during the early 1900s. The use of veins as conduits for repair or bypass of arterial vessels was reported by Goyanes (1906) and Lexer (1907).3,4 The unacceptably high thrombosis rate of these repair techniques precluded their widespread use.
DeBakey and Simeone5 published the management results of 2471 combat arterial injuries in World War II in 1946. Ligation continued to be the primary treatment during this conflict. However, 81 cases of suture repair were performed with an overall lower amputation rate, 35.8% versus 49%, when compared with ligation. This was perhaps the first indication that suture repair could be successful and improve outcome. Other factors stressed in the report included meticulous technique, wound debridement, delayed primary closure, and antibiotic use.
The Korean War began with a similar plan toward arterial injury. Patients were arriving at care facilities earlier than in any prior war because of improved evacuation techniques. An aggressive approach was then adopted, and surgical research teams were developed in both the Army and Navy to demonstrate the feasibility of acute arterial repair in wartime. Hughes (1958), Jahnke and Seeley (1953), Shannon and Howard (1955), and Spencer and Grewe (1955) then presented successful data. The amputation rate dropped to 13% in Hughes’s series after most patients had suture repair of their injuries.6
These wartime advances in vascular injury treatment crossed over into the civilian sector during the 1950s. Ferguson et al.7 reported their success with suture repair of civilian arterial injuries from 1950 to 1959. Their amputation rate of 13.6% compared well with the military experience. Early diagnosis and treatment, debridement of devitalized vessel, intimal approximation, and achievement of distal pulses were considered vital to the success of arterial repair.
The Vietnam War provided further opportunity for advancement in the treatment of acute arterial injury. A vascular registry was established at Walter Reed hospital to track soldiers with arterial injuries. The patients were again evacuated quickly, and over 98% of 1000 patients reported by Rich et al.8 underwent arterial repair. The amputation rate once again was 13.5% and consistent with the Korean War experience, despite more severe injuries encountered.
INCIDENCE
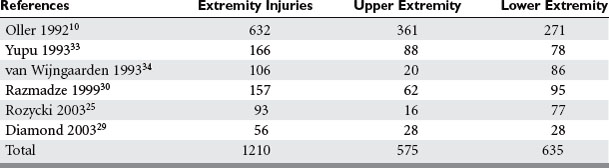
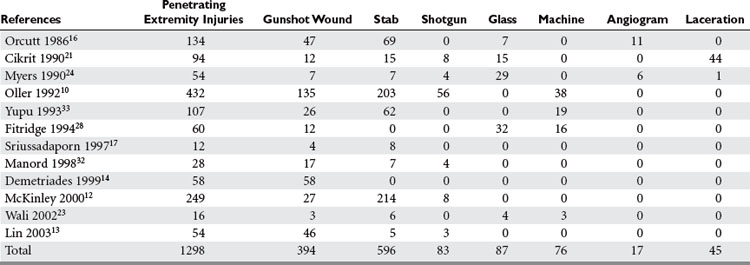
The overall incidence of vascular injury is quite low among all extremity injuries in both civilian and military settings. Beebe and DeBakey9 reported a 0.25% incidence of arterial injury among all wounds in American troops in World War I. DeBakey and Simeone5 reported a 0.96% incidence of arterial injury in World War II. The data on vascular trauma in the civilian population reported by Oller et al.10 shows a 3.7% incidence of vascular trauma among 1148 injuries in over 26,000 patients in a state registry. The overall incidence of upper extremity vascular trauma is also low. Pillai et al. reported 21 (3.3%) arterial injuries in 643 cases of upper-extremity trauma.11 However, the extremities are the most common site of vascular injury. Rich et al.8 found that 93% of all vascular injuries occurred in the extremities of American casualties in the Vietnam War. The relative incidence of vascular injury in upper versus lower extremities varies according to the series reviewed. Civilian series demonstrate upper extremity vascular injury incidence between 17% and 53%, as compared with lower extremity involvement, but when series are combined the incidence is almost equal (Table 1).
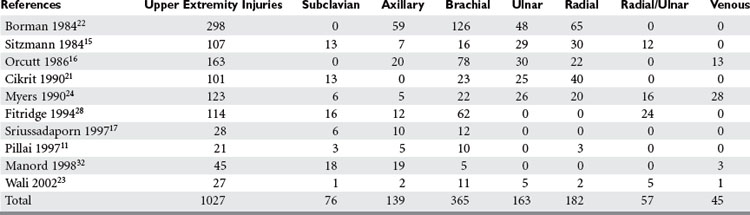
Most upper extremity vascular injuries involve the arterial system, including the subclavian, axillary, brachial, ulnar, and radial arteries (Table 2). Associated bone, nerve, and soft tissue injuries also occur and substantially impact ultimate outcome. As in much of trauma, vascular injury to the upper extremity is mainly a disease of young males. Subclavian arterial injury accounts for 3%–9% of all vascular extremity injuries. The brachial artery is the most commonly injured vessel in the upper extremity. Brachial artery injury ranges from 12%–48% of all extremity vascular traumas and radial and ulnar artery injury among all upper extremity vascular traumas is 16%–17% (see Table 2).
MECHANISM OF INJURY
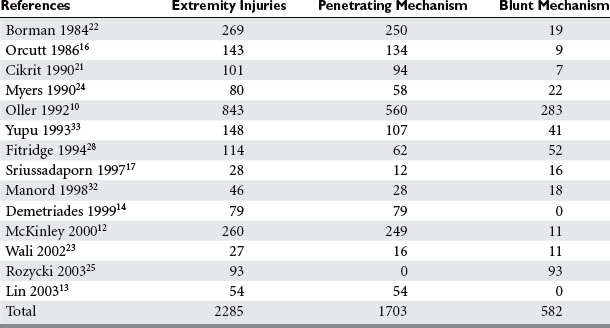
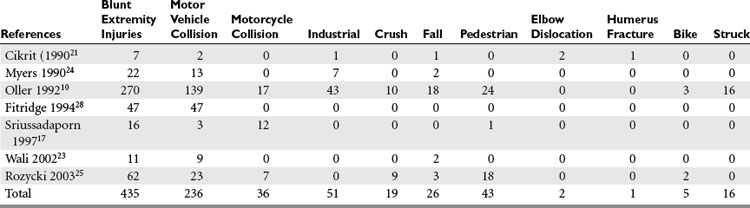
Penetrating trauma is the most common mechanism in upper extremity vascular injury. A penetrating mechanism of injury in military series is greater than 90% routinely, and consists of explosive shrapnel and high-velocity gunshot wounds. Civilian series demonstrate a penetrating mechanism for extremity vascular trauma in approximately 70%–85% of cases (Table 3). Blunt trauma comprises approximately 15%–30% of extremity vascular trauma seen in the civilian population (Table 4).
Of all penetrating extremity injuries, the most recent civilian data show stabbings to be the most common with gunshot wounds second. Other sources of penetrating trauma include glass, industrial/farming incidence, and shotgun blasts (Table 5). Military series include high-velocity mechanisms of penetrating trauma from gunshots, bombs, and mine explosions. The high-velocity mechanism of military weapons may account for more extensive associated injuries, similar to blunt trauma.
McKinley et al.12 collected data on 260 patients over a 19-year period with proximal axillary and subclavian artery injuries; only 11 patients had a blunt mechanism for their injury. In this study, stab wounds accounted for 82% of the penetrating trauma,12 while Lin et al.13 reviewed penetrating trauma to the subclavian artery and found gunshot wounds (85%) to be the most prevalent wounding agent. Demetriades et al.14 reviewed their data for subclavian and axillary penetrating injuries and again gunshots were the most common mechanism.
DIAGNOSIS
Ischemic changes were associated with absent pulses in over 70% of patients with upper extremity vascular trauma.15–17 Hematomas and bruits were less common than other hard signs. Neurologic deficits are common, but these should not always be attributed to nerve damage unless they persist after vascular repair, or a damaged nerve is directly visualized, as vascular insufficiency itself can give rise to these findings.
Noninvasive tests have been used to evaluate injured extremities for potential vascular injury, and include ankle:brachial pressure readings and duplex ultrasound. Limitations to this technology include the inability to provide the skilled individuals 24/7, and the lack of demonstrated reliability in complex injuries with large hematomas and bulky dressings. Johansen and colleagues18 have found the ankle:brachial index (ABI) to have a high sensitivity and diagnostic accuracy for vascular injury. Duplex scanning combines B-mode ultrasound and Doppler technology to allow both visual and auditory evaluation of the blood vessel. Bynoe et al.19 found duplex scanning to be both sensitive and specific when used to evaluate 198 injured extremities for vascular injury, with 20 documented cases of vascular trauma. This technique requires a highly skilled individual to perform and interpret, and limited availability of personnel and access to the extremity involved can limit its usefulness.
Conventional contrast arteriography remains the gold standard for the evaluation of injured extremities for vascular trauma, and should only be performed in hemodynamically stable patients as it requires transport of the patient to the radiology suite. With newer techniques for treatment of some vascular injuries, arteriography may also be therapeutic, allowing such interventional techniques as embolization and endovascular stenting to be performed. For many years, simple proximity of any asymptomatic injury to a major extremity artery mandated arteriography. Frykberg et al.20 demonstrated a 10.5% incidence of injury in penetrating trauma when vessel proximity to an injury was the only indication for arteriography, although all injuries were nonocclusive with a largely benign natural history.20 They concluded that in the absence of hard signs, proximity alone should not be an indication for arterial imaging or investigation. Certainly high-velocity penetrating mechanisms or complex blunt mechanisms may justify more liberal use of imaging.11 Civilian studies currently demonstrate that 18%–57% of patients with extremity vascular trauma have arteriography performed preoperatively.11,17,21–25 Complications from arteriography occur 2%–4% of the time, although major complications typically occur in far less than 1% of cases. Arteriography can also be performed more quickly, safely and cheaply in the emergency center or operating room by direct arterial injection of contrast by the surgeon.
Anatomic Location of Injury and Injury Grading
The brachial artery begins at the inferior border of the teres major and extends to just below the antecubital fossa. The first branch, the profunda, extends posteriorly through the medial and long heads of the triceps muscle along with the radial nerve to supply the posterior compartment. The superior ulnar collateral artery courses medially along with the ulnar nerve to supply a portion of the posterior compartment. A final branch, the inferior ulnar collateral, travels toward the medial epicondyle of the elbow. The median nerve follows the path of the brachial artery as it travels to the elbow. The brachial artery follows the inner border of the biceps muscle as it extends down the arm. Two brachial veins, venae comitantes, accompany the brachial artery. Extensive collateral circulation at the elbow is a result of the anastomosis of the branches of the brachial artery with inferior vessels.
The innermost layer of the artery and vein is the intima, which consists of endothelial cells. Smooth muscle and elastic fibers make up the middle layer or media and the outer layer is the adventitia, which consists of connective tissue. Forces placed on the vessels will result in a variety of injury patterns. The particular injury will influence the way the patient presents with the injury and how a diagnosis may need to be made. The force of the wounding agent will also affect the extent of the injury and the options available for treatment. High-velocity gunshot wounds can cause significant soft tissue damage and disrupt more than the primary vessels but the collaterals as well. In fact, the blast effect from high velocity weapons can damage vessels even without directly hitting them.3
Lacerations and transections are the most common types of extremity vascular injury (Figure 1). A full-thickness tear in the wall with the vessel still intact is a laceration and can often be repaired by simple lateral suture. Hemorrhage is typically more pronounced with a laceration because the smooth muscle contracts and keeps the vessel open. These injuries may present a diagnostic dilemma because the pulse may be intact distal to the injury. A transection is a full-thickness cut through the vessel with disruption of the vessel. The ends of the vessel tend to constrict and bleeding will stop. Loss of the distal pulse with ischemia to the extremity is usually evident. The extent of the surrounding tissue destruction and or vessel loss will influence treatment strategies.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree