142 Tuberculosis
 Epidemiology
Epidemiology
The World Health Organization (WHO) estimates that one-third of the world’s population is latently infected with Mycobacterium tuberculosis.1 From this pool, approximately 9 million active tuberculosis (TB) cases emerge annually, resulting in 2 million deaths and making TB the second leading cause of death by an infectious agent worldwide.2 The vast majority of TB cases (95%) occur in the developing world. Incidence rates exceed 300 cases per 100,000 persons throughout sub-Saharan Africa, the Indonesian and Philippine archipelagos, Afghanistan, Bolivia, and Peru.1,3 Areas with the most cases per year include densely populated India (2 million cases per year) and China (1.3 million cases per year).
In the United States, the TB rate continues to decline, with 3.8 new cases per 100,000 reported in 2009, the lowest rate recorded since national reporting began in 1953. Foreign-born persons and racial/ethnic minorities continue to bear a disproportionate burden of TB disease in the United States. In 2008, the TB rate within the foreign-born population in the United States was 10 times higher than in U.S.-born persons.4 TB rates among Hispanics and blacks were nearly eight times higher than among non-Hispanic whites, and rates among Asians were nearly 23 times higher than among non-Hispanic whites. Among U.S.-born racial and ethnic groups, the greatest racial disparity in TB rates was seen in the black population, who are seven times more likely to develop active TB than U.S.-born whites. Other groups at increased risk for active TB include prisoners, the homeless, and human immunodeficiency virus (HIV)-positive individuals.
The acquired immunodeficiency syndrome (AIDS) epidemic has contributed significantly to the rise in TB cases worldwide, with about 1.5 million individuals with active TB per year co-infected with HIV. HIV increases the risk of developing TB by 20.6-fold in countries where the prevalence of HIV is more than 1% in the general population.5 Co-infection with HIV contributes significantly to TB-related mortality.
 The Serious Problem of Highly Drug-Resistant Tuberculosis
The Serious Problem of Highly Drug-Resistant Tuberculosis
Drug-susceptible TB is readily curable provided adherence to medications is followed. However, drug-resistant TB requires a significantly longer course of antibiotics coupled with second-line agents that often are accompanied by difficult-to-tolerate side effects. More importantly, highly drug-resistant TB is associated with significant increase in morbidity and mortality. In the early 1990s, substantial levels of drug resistance began emerging in urban parts of the United States.6 Although the incidence of drug-resistant TB has diminished in the United States, it is increasingly problematic in many parts of the world.7
Multidrug-resistant TB (MDR-TB) is defined as resistance to two of the most powerful first-line anti-TB drugs, isoniazid (INH) and rifampin (RIF). Isolates that are resistant to multiple other combinations of anti-TB drugs but not to INH or RIF are not classified as MDR-TB. It is estimated that of the 9 million new cases of TB per year in the world, 500,000 are due to MDR-TB. Whereas drug-resistant TB is increasing at an alarming rate worldwide, particularly in India and China, prevalence in the United States decreased between 1991 and 2006 from 3.5% to 1.1%.8 MDR-TB disproportionately affects foreign-born individuals, accounting for 0.4% of TB cases occurring in U.S.-born persons and 1.3% in foreign-born individuals.9
Extensively drug-resistant tuberculosis (XDR-TB) is defined as resistance to INH, RIF, any fluoroquinolone, and to a second-line injectable (amikacin, kanamycin, or capreomycin). XDR-TB has emerged with a wide geographic distribution, including the United States, and is associated with worse treatment outcomes than MDR-TB, especially in those co-infected with HIV.7,10–15
 Pulmonary Tuberculosis
Pulmonary Tuberculosis
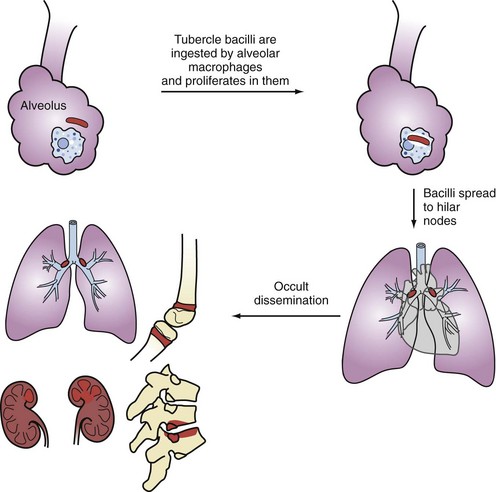
Primary infection occurs following airborne implantation of tubercle bacilli into the lungs. M. tuberculosis spreads from the lungs to hilar lymph nodes, and then throughout the bloodstream (Figure 142-1). Although primary infection is usually asymptomatic in adults, it can present with fever, hilar adenopathy, lung infiltrates, pleural effusions, and even severe pulmonary disease that may mimic viral or bacterial pneumonia, which may delay the diagnosis of TB. In severely immunocompromised patients, primary TB may be aggressive and become disseminated. Pleural TB is usually a manifestation of primary TB, although it may also occur with reactivation disease. Pleural TB can present as pleuritis or empyema. Pleural biopsy specimens are more likely to yield positive cultures than pleural fluid.
Most cases of active TB are due to reactivation of latent TB infection (LTBI). Active TB develops in about 10% of immunocompetent individuals with LTBI and tends to occur within the first 2 years of the initial infection. Typically, reactivation TB is a subacute fibrocavitary pneumonia involving the upper lobes and/or superior segments of the lower lobes. However, reactivation TB can involve any organ system and can present in a fulminant fashion with respiratory failure.16
There are some common clinical characteristics of TB patients who require ICU care. In a study of 58 ICU patients with confirmed TB, 22 (37.9%) required mechanical ventilation, and 15 (25.9%) died in the hospital.17 The factors independently associated with mortality were acute renal failure, need for mechanical ventilation, chronic pancreatitis, sepsis, acute respiratory distress syndrome (ARDS), and nosocomial pneumonia.17 Both primary and reactivation TB can cause bilateral alveolar infiltrates, hypoxic respiratory failure, and ARDS.16,18 In another study of patients hospitalized with pulmonary TB, six factors were shown to be associated with respiratory failure or death: lymphopenia, advanced age, concomitant smear-positive extrapulmonary TB, alcoholism, a high percentage of neutrophils on the peripheral white blood cell count, and lack of radiographic cavitation.19 Laboratory findings of anemia and hypoalbuminemia have been shown to be predictors for death in patients with respiratory failure due to TB.20 However, these findings are not specific to TB and commonly present in the critically ill.
Consolidation is the most frequent radiographic pattern of patients with pulmonary TB who are admitted to the ICU.21 Because this radiographic pattern is highly nonspecific, chest x-rays are often unhelpful in raising the suspicion for TB. Consolidation on initial chest radiograph has also been shown to be a strong independent risk factor for in-hospital mortality.22 One possible reason for this is a delay in the diagnosis; clinicians may be more prone to favor a diagnosis of non-tuberculous pneumonia in the absence of cavitation or miliary pattern. Another is that consolidation may be an indication of a suboptimal immune response to the infection. Pulmonary gangrene, which carries a mortality of up to 75%, can ensue when rapid progression of infiltrate causes vascular damage and death of lung tissue.23 Other life-threatening complications of pulmonary TB include hemoptysis, spontaneous pneumothoraces, bronchopleural fistulas, and empyema. Not unexpectedly, delayed recognition and treatment of nosocomial pneumonia complicating TB in patients requiring mechanical ventilation has a significant adverse effect on survival.24
Perhaps the best safeguard to prevent missing a diagnosis of pulmonary or disseminated TB in the critically ill is to maintain a high index of suspicion of it in at-risk individuals (e.g., foreign-born or immunosuppressed patients). Studies have shown that the presence of diffuse infiltrates consistent with ARDS and acute respiratory failure may cause physicians to inappropriately dismiss the diagnosis of TB.25–27 Older individuals (≥65 years) or patients with AIDS may also have delayed diagnosis of TB, due in part to atypical presentations.28,29
Hospital mortality has been reported to be 60% for patients with respiratory failure due to pulmonary TB.22 Hence, despite being a relatively rare cause of respiratory failure in ICU patients, pulmonary TB carries a poor prognosis. Early recognition of the infection is essential to reduce mortality and prevent nosocomial spread of M. tuberculosis.27
 Disseminated Tuberculosis
Disseminated Tuberculosis
Disseminated, or “miliary,” TB is more likely to occur in the very young and very old and in patients with underlying diseases such as HIV. It may result from either primary or reactivation TB. Disseminated TB typically presents subacutely with symptoms present for days to months, but it can manifest fulminantly with septic shock and multiorgan failure.30 Typical presenting signs and symptoms include fever, malaise, weight loss, dyspnea, and hypoxia.
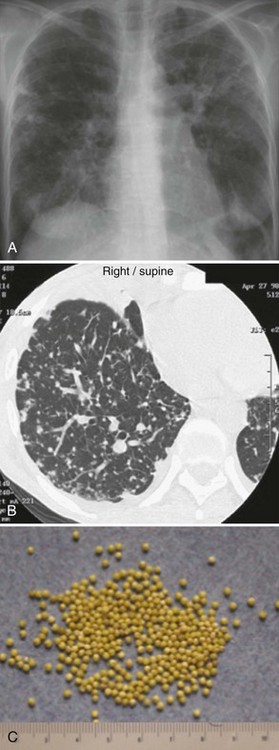
The chest radiograph (Figure 142-2, A) and computed tomography (CT) scan (see Figure 142-2, B) show a typical miliary pattern manifested by a profusion of diffuse small (<2 mm) nodules that resemble the size and uniformity of millet seeds (see Figure 142-2, C). In some cases of disseminated disease, the chest radiograph may appear normal. Virtually any organ may be involved, including the adrenals, brain, meninges, liver, pancreas, eyes, urinary tract, and skin. Bone marrow involvement by TB commonly manifests with anemia, leukemoid reaction, and thrombocytosis. The diagnosis of miliary TB can be difficult. If disseminated TB is suspected, sputum smears should be obtained even if lung disease is not apparent. Biopsy and culture of affected tissue(s), such as the bone marrow, are often required. Culture of blood, urine, and/or stool may be positive, especially in HIV-positive patients.30
 Neurologic Tuberculosis
Neurologic Tuberculosis
Tuberculous Meningitis
TB meningitis is rare in developed countries, with approximately 300 to 400 cases in the United States each year. It occurs via rupture of a subependymal tubercle that has seeded and formed during primary infection or disseminated disease. Individuals at high risk for TB meningitis include very young children with primary TB and older patients with immunodeficiency disorders such as HIV. Most patients with TB meningitis will have no known history of TB, but evidence of extrameningeal disease (e.g., pulmonary, urinary, etc.) can be found in about half of these patients.31,32 The tuberculin skin test is positive in only about 50% of patients with TB meningitis.
TB meningitis is typically a subacute disease. In one review of 58 cases, symptoms were present for 1 day to 9 months, with a median of 10 days prior to diagnosis.31 A prodromal phase of low-grade fever, malaise, headache, dizziness, vomiting, and/or personality changes may persist for 2 to 3 weeks before the patient presents for medical care. Typical findings at presentation include severe headache, altered mental status, stroke, hydrocephalus, and cranial neuropathies. These clinical features are the result of basilar meningeal fibrosis and vascular inflammation.33 Classic features of bacterial meningitis such as stiff neck and fever may be absent. When allowed to progress, coma and seizures may ensue.
The diagnosis of TB meningitis can be difficult and may be based only on clinical findings without definitive microbiological proof. Certain clinical characteristics such as longer duration of symptoms (>6 days), moderate cerebrospinal fluid (CSF) pleocytosis, and the presence of focal deficits increase the probability of TB meningitis.34,35 Characteristic CSF findings of TB meningitis include:
CSF samples should be sent for acid-fast smears, but this is associated with low sensitivity (<20%). Large volumes (10-15 mL) from several daily lumbar punctures are often needed for a microbiological diagnosis. Sensitivity is increased if four spinal taps are performed. Culture can take weeks and is also associated with low sensitivity. Stereotactic biopsy can be performed if tissue samples are needed. Mycobacterial antigens by enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay have been detected in the CSF of patients with TB meningitis.36
Recent meta-analysis calculated that commercial nucleic acid amplification (NAA) assays used for the diagnosis of TB meningitis were 56% sensitive and 98% specific.37,38 Unfortunately, considerable variability in sensitivity and specificity among tests from different laboratories makes it more difficult to interpret results. Most studies conclude that commercial NAA tests can confirm TB meningitis but cannot rule it out.39 Thus a negative test neither excludes the diagnosis nor obviates the need for continued empirical therapy if the clinical suspicion is high. Comparisons of NAA and microscopy/culture using large volumes of CSF have indicated that the sensitivity of microscopy was similar to NAA for the diagnosis of TB meningitis, and repeated testing gave the highest diagnostic yield.40 The sensitivity of CSF microscopy and culture falls rapidly after the start of treatment, whereas mycobacterial DNA may remain detectable within the CSF up to a month after the start of treatment.41
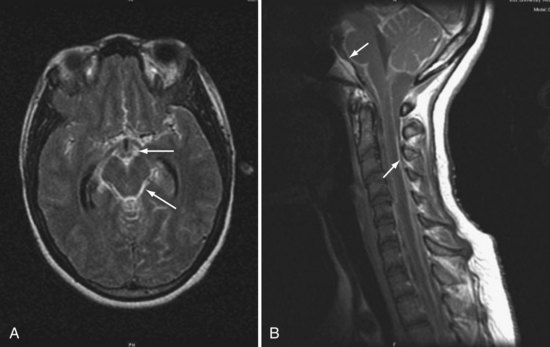
Magnetic resonance imaging (MRI) often reveals basilar meningeal enhancement (Figure 142-3) and/or hydrocephalus.32 Hypodensities due to cerebral infarcts, and ring or nodular enhancing lesions can also be seen. MRI is superior to CT for evaluating the brainstem and the extent of lesions.
The outcome of TB meningitis is improved by timely treatment. Thus empirical treatment is warranted when risk factors and clinical features are suggestive of this diagnosis, even before microbiological confirmation. Chemotherapy for TB meningitis follows the model of short-course chemotherapy for pulmonary TB—an induction phase followed by a continuation phase. But unlike pulmonary TB, the optimal drug regimen and duration of each phase of treatment are not clearly established. INH and RIF remain the most essential drugs. INH penetrates the CSF freely and has potent early bactericidal activity.42–44 RIF penetrates the CSF less well (maximum concentrations around 30% of plasma), but the high mortality from RIF-resistant TB meningitis has confirmed its central role in the treatment of CNS disease.45 INH, RIF, and pyrazinamide are considered mandatory at the beginning of TB meningitis treatment, and some centers use all three drugs for the duration of therapy.46 There are no data from controlled trials to guide choice of the fourth drug. Most authorities recommend either streptomycin or ethambutol, although neither penetrates the CSF well in the absence of inflammation, and both can produce significant adverse reactions. Therapy should be continued for 9 to 12 months.
Adjunctive corticosteroid treatment of TB meningitis has been recommended for more than 50 years, but there has been long-standing concern that corticosteroids may reduce the penetration of anti-TB drugs into the CNS.33 A recent Cochrane systematic review and meta-analysis of 7 randomized controlled trials involving 1140 participants (with 411 deaths) concluded that corticosteroids improved outcome in HIV-negative children and adults with TB meningitis, but the benefit in HIV infected individuals remains uncertain.47 The results were heavily influenced by a study performed in 545 Vietnamese adults with TB meningitis which observed that treatment with dexamethasone was associated with a significantly reduced risk of death.48 However, there was no demonstrable improvement in the combined endpoint of death or severe disability at 9-month follow-up. The survival benefit associated with corticosteroid therapy may have been due in part to a reduction in severe adverse events (9.5% versus 16%), particularly hepatitis, that necessitated changes in anti-TB drug regimens. No mortality benefit from dexamethasone was evident in 98 HIV-infected patients included in the study.48
Because there are no controlled trials comparing different corticosteroid regimens, the choice should be based on those found to be effective in published trials. One recommended regimen for adults is dexamethasone, 12 mg a day for 3 weeks, followed by gradual taper over the next 3 weeks.49 In the large study from Vietnam, patients with mild disease received intravenous (IV) dexamethasone, 0.3 mg/kg/d × 1 week, 0.2 mg/kg/d × 1 week, and then 4 weeks of tapering oral therapy.48 For patients with more severe TB meningitis, IV dexamethasone was given for 4 weeks (1 week each of 0.4 mg/kg/d, 0.3 mg/kg/d, 0.2 mg/kg/d, and 0.1 mg/kg/d), followed by 4 weeks of tapering oral dexamethasone therapy.48
Prognosis of TB meningitis largely depends on neurologic status at the time of presentation and time to treatment initiation. Most patients will die in 5 to 8 weeks if not treated. Various case series indicate a mortality rate between 7% and 65% in developed countries and up to 69% in underdeveloped areas.31,32,50 Neurologic sequelae occur in up to 50% of survivors.50 Mortality risk is highest in those with comorbidities, severe neurologic involvement on admission, rapid progression of disease, and being elderly.
Other Central Nervous System Manifestations of Tuberculosis
Other CNS manifestations of TB include brain abscesses, intracranial tuberculomas, vasculitis, radiculomyelitis, and spinal arachnoiditis. These can occur in conjunction with TB meningitis but are less likely to be seen as isolated findings in the ICU. Intracranial tuberculomas are more common among pediatric patients, especially infants, and can occur in any region of the brain. They result from hematogenous spread of TB. Tuberculous radiculomyelitis is a paradoxical reaction to the treatment of TB meningitis and may respond to corticosteroids. Signs and symptoms include subacute paraparesis, radicular pain, bladder disturbance, and paralysis.51
 Cardiovascular Tuberculosis
Cardiovascular Tuberculosis
Tuberculous Pericarditis
The diagnosis of TB pericarditis can be difficult to prove. Culture of pericardial fluid is positive in only 30% of cases, and pericardial biopsy has a yield of approximately 60%. Biopsy of the pericardium may reveal granulomatous changes consistent with TB or stains positive for acid-fast bacteria. The presence of elevated adenosine deaminase levels in the pericardial fluid has been shown to indicate TB pericarditis, but confirmation is needed.52 PCR holds promise as a more sensitive test in diagnosis of TB pericarditis.53 Many individuals are treated empirically for TB pericarditis based on clinical suspicion, positive tuberculin skin test, imaging studies, and exudative pericardial fluid with high protein and mononuclear white count. Treatment involves standard four-drug regimens as for other manifestations of TB. Prednisone, 60 mg a day, tapered over 11 weeks, is sometimes used in addition to anti-TB therapy, and has been shown to reduce the need for operative intervention.54 Pericardiectomy is sometimes necessary in the treatment of refractory or recurrent disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree