Trauma and Burns
Levon M. Capan
Sanford M. Miller
Kevin J. Gingrich
Key Points
Related Matter
Management of the Major Trauma Patient
Nail Trachea Case
Pneumothorax
Injury is responsible for 9% of the total annual mortality (5 million people) in the world.1 Traffic accidents alone killed 3,500 people every day in 2009; almost half of the victims were pedestrians, cyclists, and motorcyclists.2 Violence, encompassing homicides, suicides, and war-related injuries, is also estimated to kill about 1.5 million people every year in the world of whom the vast majority reside in low- and middle-income countries.3
In the United States, according to data from the National Safety Council intentional injuries (suicide, homicide, and assault) claimed 55,000 lives in 2008, while in 2010, unintentional (motor vehicle, falls, drowning, poisoning, etc.) mortality claimed 126,000 lives. Trauma, with an estimated 181,000 deaths per year, was the third leading cause of death after heart disease and cancer. Unintentional injuries were the fifth, suicides the tenth, and assault the fifteenth leading causes of death overall. For the age range between 15 and 31, accidents, suicide, and homicide were the three leading causes of death. Morbidity caused by injuries is far in excess of mortality; in 2010, a total of 37.9 million emergency room visits were related to unintentional injuries. The estimated cost of unintentional injuries alone that year was $731 billion, including the costs of fatal and nonfatal injuries, employer costs, vehicle damage, and fire losses.4
Approximately 75% of the hospital deaths from high-energy trauma such as motor vehicle accidents, falls, and gunshot or stab wounds occur within 48 hours after admission, most commonly from central nervous system (CNS), thoracic, abdominal, retroperitoneal, or vascular injuries.5 CNS injury and hemorrhage are the most common causes of early trauma mortality.6 Nearly one-third of these patients die within the first 4 hours after admission, representing the majority of operating room (OR) trauma deaths. Of the hospital deaths, 5% to 10% occur between the third and seventh day of admission, usually from CNS injuries, and the rest in subsequent weeks, most commonly as a result of multiorgan failure.5 Pulmonary thromboembolism and infectious complications may also contribute to mortality during this phase.5 Interestingly, injuries caused by low energy impacts, mainly from falls usually in the elderly, also produce significant mortality from head injury and complications of skeletal injuries. Of these deaths, 20% occur within 48 hours, 32% after 3 to 7 days, and 48% after 7 days. Pre-existing conditions such as congestive heart failure, cirrhosis, warfarin, and/or β-blocker usage increase the mortality rate in trauma patients.7
Initial Evaluation and Resuscitation
The secondary survey involves a more elaborate systematic examination of the entire body to identify additional injuries. Radiographic and other diagnostic procedures may also be performed if the stability of the patient permits. Within this general framework the anesthesiologist, aside from managing the airway, contributes as part of the team for evaluation and resuscitation, while gathering information needed for possible future anesthetic management.
Injuries may be missed during initial evaluation and even during emergency surgery, resulting in significant pain, complications, residual disability, delay of treatment, or death.8 Reported missed diagnoses include cervical spine, thoracoabdominal, pelvic, nerve, and external soft tissue injuries, and extremity fractures. Some of these injuries may present during administration of anesthesia, such as spinal cord damage in a patient with unrecognized cervical spine injury, massive intraoperative bleeding from an unrecognized thoracoabdominal injury during extremity surgery, or sudden intraoperative hypoxemia in a patient with unrecognized pneumothorax.
A tertiary survey within the first 24 hours after admission (which may include a period of anesthesia) can potentially diagnose the majority of clinically significant injuries missed during initial evaluation by repeating the primary and secondary examinations and reviewing the results of radiologic and laboratory testing.8
Airway Evaluation and Intervention
Airway Obstruction
displaced bone or cartilage fragments. Bleeding into the cervical region may produce airway obstruction not only because of compression by the hematoma, but also from venous congestion and upper airway edema as a result of compression of neck veins. Signs of upper and lower airway obstruction include dyspnea, cyanosis, hoarseness, stridor, dysphonia, subcutaneous emphysema, and hemoptysis. Cervical deformity, edema, crepitation, tracheal tug and/or deviation, or jugular venous distention may be present before these symptoms appear and may help indicate that specialized techniques are required to secure the airway.
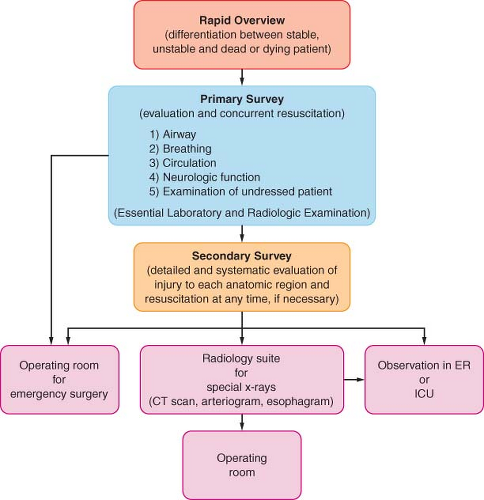 Figure 52.1. Clinical sequence for initial management of the major trauma patient. CT, computed tomography; ER, emergency room; ICU, intensive care unit. |
The initial steps in airway management are chin lift, jaw thrust, clearing of the oropharynx, placement of an oropharyngeal or nasopharyngeal airway, and, in inadequately breathing patients, ventilation with a self-inflating bag. Immobilization of the cervical spine and administration of oxygen should be applied simultaneously. Blind passage of a nasopharyngeal airway or a nasogastric or nasotracheal tube should be avoided if a basilar skull fracture is suspected because it may enter the anterior cranial fossa. A cuffed oropharyngeal airway (Combitube or King’s airway) or a laryngeal mask airway (LMA) may permit ventilation with a self-inflating bag, although neither provides protection against aspiration of gastric contents. They may be used as temporary measures and can serve as a bridge for a brief period to re-establish the airway patency or to facilitate intubation aided by a flexible fiberoptic bronchoscope (FOB). If they do not provide adequate ventilation, the trachea must be intubated immediately using either direct laryngoscopy or a cricothyroidotomy, depending on the results of airway assessment.
Maxillofacial, neck, and chest injuries, as well as cervicofacial burns, are the most common trauma-related causes of difficult tracheal intubation. Airway assessment should include a rapid examination of the anterior neck for feasibility of access to the cricothyroid membrane. Tracheostomy is not desirable during initial management because it takes longer to perform than a cricothyroidotomy and requires neck extension, which may cause or exacerbate cord trauma in patients with cervical spine injuries. Conversion to a tracheostomy should be considered later to prevent laryngeal damage if a cricothyroidotomy will be in place for more than 2 to 3 days. Possible contraindications to cricothyroidotomy include age younger than 12 years and suspected laryngeal trauma. Permanent laryngeal damage may result in the former, and uncorrectable airway obstruction may occur in the latter situation.
Full Stomach
A full stomach is a background condition in acute trauma: the urgency of securing the airway often does not permit adequate time for pharmacologic measures to reduce gastric volume and acidity. Thus, rather than relying on these agents, the emphasis should be placed on selection of a safe technique for securing the airway when necessary: rapid-sequence induction with cricoid pressure for those patients without serious airway problems, and awake intubation with sedation and topical anesthesia, if possible, for those with anticipated serious airway difficulties.
In agitated and uncooperative patients, topical anesthesia of the airway may be impossible, whereas administration of sedative agents may result in apnea or airway obstruction, with an increased risk of aspiration of gastric contents and inadequate conditions for tracheal intubation. After locating the cricothyroid membrane and denitrogenating the lungs, a rapid-sequence induction may be used to allow securing of the airway with direct laryngoscopy or, if necessary, immediate cricothyroidotomy. Personnel and material necessary to perform translaryngeal ventilation or cricothyroidotomy must be in place before induction of general anesthesia.
Head, Open Eye, and Contained Major Vessel Injuries
The principles of tracheal intubation are similar for these injuries. Apart from the need to ensure adequate oxygenation and ventilation, these patients require deep anesthesia and profound muscle relaxation before airway manipulation. This helps prevent hypertension, coughing, and bucking, and thereby minimizes intracranial, intraocular, or intravascular pressure elevation, which can result in herniation of the brain, extrusion of eye contents, or dislodgment of a hemostatic clot from an injured vessel, respectively. The preferred anesthetic sequence to achieve this goal in patients who are not hemodynamically compromised includes preoxygenation and opioid loading, followed by relatively large doses of an intravenous anesthetic and muscle relaxant. Hemodynamic responses to this sequence should be carefully monitored and promptly corrected. Systemic hypotension, intracranial pressure (ICP) elevation, and decreased cerebral perfusion pressure (CPP; CPP equals mean arterial pressure minus ICP) may occur whether cerebral autoregulation is present or absent in patients with head injuries, and if untreated it can produce secondary ischemic insults. Ketamine is probably contraindicated in patients with head and vascular injuries because it may increase both intracranial and systemic vascular pressures. However, no significant increase in intraocular pressure (IOP) has been documented. Any muscle relaxant, including succinylcholine, may be used as long as the fasciculation produced by this agent is inhibited by prior administration of an adequate dose of a nondepolarizing muscle relaxant. Alternatively, rocuronium can provide intubating conditions within 60 seconds with a dose of 1.2 to 1.5 mg/kg; neuromuscular blockade produced by this dose lasts approximately 2 hours. Of course, neither muscle relaxants nor intravenous anesthetics are indicated when initial assessment suggests a difficult airway. As in any other trauma patient, hypotension dictates either reduced or no intravenous anesthetic administration.
Cervical Spine Injury
Overall, 2% to 4% of blunt trauma patients have cervical spine (C-spine) injuries, of which 7% to 15% are unstable.10 The most common causes include high-speed motor vehicle accidents, falls, diving accidents, and gunshot wounds. Head injuries, especially those with low Glasgow coma scores (GCS) and focal neurologic deficits, are likely to be associated with C-spine injuries. Approximately 2% to 10% of head trauma victims have C-spine injuries, while 25% to 50% of patients with C-spine injuries have an associated head injury.10 The incidence of assault-related injuries depends on the mechanism, being highest after gunshot wounds (1.35%), lowest after stab wounds (0.12%), and intermediate after blunt trauma (0.4%) to the cervicothoracic region.
Initial Evaluation
Accurate and timely evaluation is important because 2% to 10% of blunt trauma induced C-spine injury patients develop new or worsening neurologic deficits after admission, partly attributable to delayed diagnosis and improper C-spine protection and/or manipulation.10 Often there is no time to evaluate the injury, and emergency airway management may have to be performed without ruling out C-spine injury while the patients are in a rigid collar and neck stabilizing devices. Clearance of the neck at the earliest possible time after airway management should be performed to minimize the complications associated with the collar, such as pressure ulceration, ICP elevation in head injured patients, compromised central venous access, and airway management challenges if reintubation is needed.
In the conscious patient with a suspected injury, diagnosis is relatively easy. According to the American National Emergency X-Radiography Utilization Study (NEXUS), a clinical evaluation revealing no posterior midline tenderness in the neck and no focal neurologic deficit in an injured patient with a normal level of alertness, no evidence of intoxication, and absence of painful distracting injury indicates a low probability of a C-spine injury, and there is thus no need for radiographic evaluation.11 Recently, however, it has been shown that a significant number of major trauma patients cleared by these criteria had clinically important unstable C-spine injuries requiring treatment. Therefore, routine computerized tomography (CT) in addition to clinical evaluation is recommended to rule out C-spine injury in major trauma victims.12 Probably the reason for the lower reliability of the NEXUS criteria is difficulty in evaluating distracting injuries.
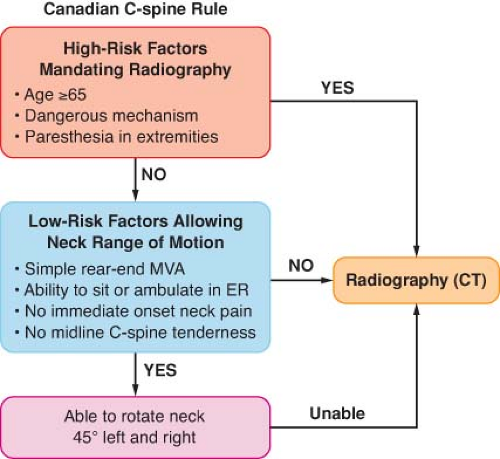
The Canadian C-spine rule for radiography after trauma is another tool designed to identify low-risk patients.10 With this diagnostic tool, proper answers to the following three questions eliminate the possibility of injury and the need for radiographic studies: (a) Is there any high-risk factor mandating radiography? (b) Are there low-risk factors that permit safe evaluation of the range of motion of the neck? (c) Can the patient rotate the neck laterally for 45 degrees in each direction without pain? (Fig. 52-2).
Comparison of these two sets of criteria showed that the Canadian rule is more reliable than those for NEXUS in diagnosing C-spine injury in responsive patients.13
Comparison of these two sets of criteria showed that the Canadian rule is more reliable than those for NEXUS in diagnosing C-spine injury in responsive patients.13
Magnetic resonance imaging (MRI) is a reliable tool; a normal examination can conclusively exclude C-spine injury.15 It is thus the gold standard for ruling out C-spine injury. However, it is so sensitive that it can detect subtle injuries that are clinically insignificant. It cannot be performed in multiple trauma patients who have metallic skeletal fixators. It is expensive and requires patient transport. Finally, it cannot be performed in the first few days after injury, the period when airway management is most commonly performed. A more recently proposed approach, which is practiced in many countries and in many, but not all, trauma centers in the United States, is to rely on the CT study performed using multidetector devices with <3-mm cuts. The diagnostic capability of this method is excellent, with the possibility of missing 1 unstable C-spine injury in about 5,000 patients not cleared by clinical examination.16
There are certain situations that theoretically could be missed with CT examination. Acute rupture of a transverse atlantal ligament produces significant instability despite the absence of a neurologic deficit and normal alignment while the patient is supine on a CT scanner. Likewise, in a head-injured patient, a mild to moderate central cord syndrome, which is referred to as spinal cord injury without radiological abnormality, may be difficult to discern with a CT scan. Familiarity with these diagnostic strategies may help in the assessment of patients with C-spine injury before airway management.
Interestingly, published series describe very few instances of neurologic deficits related to airway management in C-spine–injured patients. Recently, Hindman et al.17 reviewed the closed claims data for perioperative cervical cord, nerve root, and spine injury between 1970 and 2007, which showed that overall airway management–related neurologic damage represented 11% of 48 claims. Nine patients in the series had unstable spines preoperatively and developed neurologic deficits. In two of these patients the injury was attributed to airway management following direct laryngoscopy and intubation without C-spine precautions. McLeod and Calder18 reviewed nine allegedly intubation-related cervical spinal cord injuries. Of these, three patients in two reports developed increased neurologic deficit after laryngoscopy and intubation without stabilization of the neck. It is likely that two of these patients are the same patients described by Hindman et al.17 Thus it is possible that airway management–related cervical cord injury in C-spine injured patients can occur, but if it does, it is rare.
Airway Management
Almost all airway maneuvers, including jaw thrust, chin lift, head tilt, and oral airway placement, result in some degree of C-spine movement.10 To secure the airway with direct laryngoscopy, manual in-line stabilization (MILS) of the neck is the standard care of these patients in the acute stage. A hard cervical collar alone, which is routinely placed, does not provide absolute protection, especially for rotational movements of the neck. MILS is best accomplished by having two operators in addition to the physician who is actually managing the airway. The first operator stabilizes and aligns the head in neutral position without applying cephalad traction. The second operator stabilizes both shoulders by holding them against the table or stretcher. The anterior portion of the hard collar, which limits mouth opening, may be removed after immobilization.
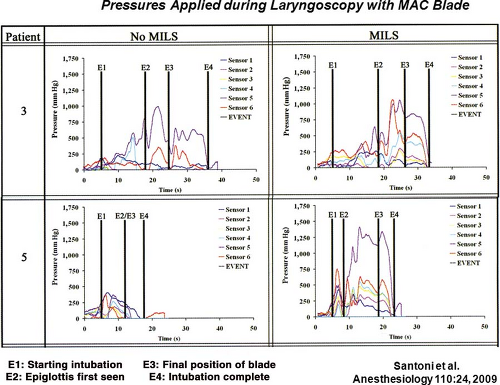
In the presence of MILS, the glottic view may be suboptimal in 10% to 15% of patients during direct laryngoscopy because of limitation of neck extension. Airway management may be further compromised in some patients because of enlargement of the prevertebral space by a hematoma from the vertebral fracture. Consequently, greater anterior pressure needs to be applied to the tongue by the laryngoscope blade to visualize the larynx. This increased anterior pressure is transmitted to the spine and can increase the movement of the unstable vertebral segment. Thus, the more the restriction of the glottic view during direct laryngoscopy, the greater the pressure on the tongue, the spine, and the unstable segment with potential displacement of the unstable fragment. Santoni et al.19 demonstrated that during various phases of direct laryngoscopy and intubation, the pressures exerted on the tongue and indirectly to the spine were greater with MILS than without MILS (Fig. 52-3). This finding confirmed the results of a videofluoroscopic study by Lennarson et al.20 who demonstrated significant anteroposterior displacement when MILS was applied to cadavers with destabilized C-spines.
Although convincing, these data should not eliminate the current standard practice of applying MILS during airway management of these patients. Currently, there is no scientifically rigorous clinical trial to show conclusively that airway management without MILS is associated with a favorable spinal cord outcome. Based on the available data, it is, however, reasonable to allow some relaxation of the MILS to improve the glottic view when visualization of the larynx is restricted.
Other measures and techniques, including the McCoy laryngoscope, rigid fiberoptic video laryngoscopes (Glidescope, Verathon, Bothell, Washington; Airtraq, Airtraq LLC, Fenton, Missouri; McGrath, LMA North America, San Diego, California), FOB, lightwand, translaryngeal (retrograde) intubation, and cricothyroidotomy, can be used to secure the airway in the acute phase in patients requiring cervical spine immobilization. So far the existing data suggest that neck motion with modern video laryngoscopes is similar to that produced by the Macintosh blade, although they provide better glottic views. A gum elastic bougie passed through the endotracheal tube, or a satin-sheathed stylet placed through its Murphy aperture, may also be helpful. They can be inserted through the larynx more easily than the tube itself because their small diameter does not block the view of the glottis during direct laryngoscopy. Cricoid pressure may optimize the view, but it should be applied with great care as it may produce undue motion of the unstable spine if excessive force is used. Supraglottic intubating airways with or without the aid of FOB can be used, but neck movement with these devices appears to be comparable to that produced by conventional laryngoscopes. Flexible fiberoptic laryngoscopy, lightwand, and possibly translaryngeal-guided intubation (see “Maxillofacial Injuries” section) cause almost no neck movement, but blood or secretions in the airway, a long preparation time, and difficulty in their use in comatose, uncooperative, or anesthetized patients reduce their utility during initial management. Nasotracheal intubation carries the risks of epistaxis, failure
of intubation, and the possibility of entry of the endotracheal tube into the cranial vault or the orbit if there is damage to the cranial base or the maxillofacial complex. Absence of the usual signs of cranial base fracture (battle sign, raccoon eyes, or bleeding from the ear or the nose) cannot be relied on to exclude the possibility of its occurrence because with rapid prehospital transport, these signs may not be immediately apparent.
of intubation, and the possibility of entry of the endotracheal tube into the cranial vault or the orbit if there is damage to the cranial base or the maxillofacial complex. Absence of the usual signs of cranial base fracture (battle sign, raccoon eyes, or bleeding from the ear or the nose) cannot be relied on to exclude the possibility of its occurrence because with rapid prehospital transport, these signs may not be immediately apparent.
In the subacute phase of C-spine injury when time constraints, full stomach, and patient cooperation issues do not exist, the use of FOB in the awake, sedated patient with appropriate topical anesthesia is preferred. Advantages of this technique are minimal movement of the neck, positioning of the patient awake, maintenance of protective reflexes, and the ability to assess the neurologic status after intubation.
Direct Airway Injuries
Direct airway damage can occur anywhere between the nasopharynx and the bronchi. Sometimes more than one site may be involved, resulting in persistent airway dysfunction after one of the problems is corrected.21 Head, face, and neck injuries are more common in military personnel in combat than in the civilian population; effective torso protection by body armor used in combat leaves these regions unprotected.22
Maxillofacial Injuries
In addition to soft tissue edema of the pharynx and peripharyngeal hematoma, blood or debris in the oropharynx may be responsible for partial or complete airway obstruction in the acute stage of these injuries. Occasionally, teeth or foreign bodies in the pharynx may be aspirated into the airway causing some degree of obstruction, which may occur or be recognized only during attempts at tracheal intubation. Another problem is the dynamic nature of soft tissue injuries in this region. A hematoma or edema in the face, tongue, or neck may expand during the first several hours after injury and ultimately occlude the airway. Serious airway compromise may develop within a few hours in up to
50% of patients with major penetrating facial injuries or multiple trauma, caused by progressive inflammation or edema resulting from liberal administration of fluids.
50% of patients with major penetrating facial injuries or multiple trauma, caused by progressive inflammation or edema resulting from liberal administration of fluids.
The face, head, and neck are vulnerable to missile and explosion injuries.22 Although rare, massive hemorrhage, most frequently from the internal maxillary artery or its branches, and less frequently from the facial, external carotid, or sphenopalatine arteries and other small branches, may be life threatening, requiring anterior, posterior, or anteroposterior packing, intermaxillary fixation, and, when these measures are ineffective, angioembolization.23,24 Tracheal intubation or a surgical airway is necessary as an initial measure to avert airway compromise in these circumstances.
Fracture-induced encroachment on the airway or limitation of mandibular movement, pain, and trismus may limit mouth opening. Fentanyl in titrated doses of up to 2 to 4 μg/kg over a period of 10 to 20 minutes may produce an improvement in the patient’s ability to open the mouth if mechanical limitation is not present.
The selection of an airway management technique in the presence of a maxillofacial fracture is based on the patient’s presenting condition. Most patients with isolated facial injuries do not require emergency tracheal intubation. Surgery may be delayed for as long as a week with no adverse effect on the repair. Patients who present with airway compromise may be intubated using direct laryngoscopy; the decision about the use of anesthetics and muscle relaxants is based on the results of airway evaluation. When there is bleeding into the oropharynx, a flexible fiberoptic laryngoscope may be useless because of obstruction of the view. A retrograde technique, using a wire or epidural catheter passed through a 14-gauge catheter introduced into the trachea through the cricothyroid membrane, may be used if the patient can open his or her mouth. A surgical airway is indicated when there is airway compromise, when direct laryngoscopy has failed or is considered impossible, when the jaws will be wired, or when a tracheostomy will be performed anyway after definitive repair of the fracture.25 Tracheostomy may be indicated as an emergency procedure in the emergency room within a few minutes of arrival, as a delayed procedure in the OR for airway control within 12 hours of arrival, or as an elective procedure during definitive surgery in the OR more than 12 hours following admission to the hospital.22 Comminuted mandibular, midfacial bilateral LeFort III, and panfacial fractures are likely to be managed with tracheostomy for definitive surgery.25 To avoid the possible complications of tracheostomy, submental or submandibular intubation, which involves externalizing the proximal end of an orotracheal flexible armored tube through a small submental incision has been performed. Thus, the trachea remains surgically intact.26 Nasogastric or nasotracheal intubation should be avoided when a basilar skull or maxillary fracture is suspected because of the possibility that the tube may enter the cranium or the orbit. Hemorrhagic shock and life-threatening cranial, laryngotracheal, thoracic, and cervical spine injuries may accompany major facial fractures, and airway management must be tailored accordingly. The likelihood of cranial injury increases in midface fractures involving the frontal sinus, as well as the orbitozygomatic and orbitoethmoid complexes.
Cervical Airway Injuries
The strategy for tracheal intubation depends on the clinical presentation.29 The tracheas of some patients with penetrating airway injuries, especially stab wounds, may be intubated through the airway defect without the need for anesthetics or optical equipment. The presence of cartilaginous fractures or mucosal abnormalities necessitates awake intubation with an FOB or awake tracheostomy.21 Laryngeal damage precludes cricothyroidotomy. Tracheostomy should be performed with extreme caution because up to 70% of patients with blunt laryngeal injuries may have an associated cervical spine injury.29 Uncooperative or confused patients may not tolerate awake airway manipulation. It may be best to transport these patients to the OR, induce anesthesia with inhalational agents, and intubate the trachea without muscle relaxants.29 Episodes of airway obstruction during spontaneous breathing under an inhalational anesthetic can be managed by positioning the patient upright in addition to the usual maneuvers.
Table 52-1. Classification of Laryngeal Injuries | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Complete transection of the trachea is rare but when it occurs it is life threatening. The distal segment of the trachea retracts into the chest, causing airway obstruction either spontaneously or during airway manipulation. Surgery involves pulling up the distal end and performing an end-to-end anastomosis to the proximal segment or suturing it to the skin as a permanent tracheostomy. In extreme situations, such as complete or near-complete transection of the larynx and trachea, femorofemoral bypass or percutaneous cardiopulmonary support may be considered if time permits.32
Thoracic Airway Injuries
Whereas penetrating trauma can cause damage to any segment of the intrathoracic airway, blunt injury usually involves the posterior membranous portion of the trachea and the mainstem bronchi, usually within approximately 3 cm of the carina. A significant number of these injuries result from iatrogenic causes such as tracheal intubation.33 Pneumothorax, pneumomediastinum, pneumopericardium, subcutaneous emphysema, and a continuous air leak from the chest tube are the usual signs of this injury. They occur frequently but are not specific for thoracic airway damage. In patients intubated without the suspicion of a tracheal injury, difficulty in obtaining a seal around the endotracheal tube or the presence on a chest radiograph of a large radiolucent area in the trachea corresponding to the cuff suggests a perforated airway. Other radiographic findings include a radiolucent line along the prevertebral fascia due to air tracking up from the mediastinum, peribronchial air or sudden obstruction along an air-filled bronchus, and the “dropped lung” sign when complete intrapleural bronchial transection causes the apex of the collapsed lung to descend to the level of the hilum. Occasionally, simultaneous esophageal injury with a tracheoesophageal fistula may be present.34
Airway management is similar to that of cervical airway injury. Anesthetics, and especially muscle relaxants, may produce irreversible obstruction, presumably because of relaxation of structures that maintain airway patency in the awake patient. However, airway loss may also occur during attempts at awake intubation, often as a result of further distortion of the airway by the endotracheal tube, patient agitation, or rebleeding into the airway. After intubation of the trachea, the adequacy of airway intervention is evaluated mainly by auscultation and capnography. Pulmonary contusion, atelectasis, diaphragmatic rupture with thoracic migration of the abdominal contents, and pneumothorax may complicate the interpretation of chest auscultation. Likewise, carbon dioxide (CO2) elimination may be decreased or absent in shock and cardiac arrest.
The outcome after surgical repair of these injuries is often suboptimal and complicated by stump leak and empyema, suture line stenosis, or the need for tracheostomy or pneumonectomy. The recent trend is selective conservative management with an endotracheal tube placed using bronchoscopic guidance distal to the tracheal injury.35 Patients with lesions >4 cm, cartilaginous rather than membranous injuries, concomitant esophageal trauma, progressive subcutaneous emphysema, severe dyspnea requiring intubation and ventilation, difficulty with mechanical ventilation, pneumothorax with an air leak through the chest drains, and mediastinitis are still managed surgically. Those without these problems may be treated nonoperatively with a reasonable outcome.33,35
Management of Breathing Abnormalities
Of the several causes that may alter respiration after trauma, tension pneumothorax, flail chest, and open pneumothorax are immediate threats to the patient’s life and therefore require rapid diagnosis and treatment. Hemothorax, closed pneumothorax, pulmonary contusion, diaphragmatic rupture with herniation of abdominal contents into the thorax, and atelectasis from a mucous plug, aspiration, or chest wall splinting can also interfere with breathing and pulmonary gas exchange and deteriorate into life-threatening complications.
Although cyanosis, tachypnea, hypotension, neck vein distention, tracheal deviation, and diminished breath sounds on the affected side are the classic signs of tension pneumothorax, neck vein distention may be absent in hypovolemic patients and tracheal deviation may be difficult to appreciate. Diagnosis with an ultrasound probe placed on the second intercostal space on the suspected side to search for lung sliding and comet tail signs has the potential to provide rapid diagnosis; absence of these signs suggests the presence of pneumothorax.36 Inability to position most trauma patients upright and the likelihood of inadequate imaging decrease the diagnostic value of chest radiographs.37 In the supine position the “deep sulcus sign,” which results from the tendency of pleural air to track in the lateral and caudal regions, is usually the diagnostic chest radiographic sign of tension pneumothorax.37 The definitive diagnosis is made by CT scanning.36 However, in hypoxemic and hypotensive patients, immediate insertion of a 14-gauge angiocatheter through the fourth intercostal space in the midaxillary line or, at times, through the second intercostal space at the midclavicular line, is essential. There is no time for radiologic confirmation in this setting.
Pulmonary contusion, respiratory insufficiency or failure despite adequate analgesia, clinical evidence of severe shock, associated severe head injury or injury requiring surgery, airway
obstruction, and significant pre-existing chronic pulmonary disease are indications for tracheal intubation and mechanical ventilation. Outcome in these patients may be dependent on the pattern of ventilation. In head injury patients, unless the clinical evidence suggests imminent cerebral herniation, hyperventilation must be avoided because it increases cerebral vasoconstriction, thus decreasing perfusion with accumulation of cerebral lactic acid immediately after its institution.42 In hypovolemic patients, hyperventilation may interfere with venous return and cardiac output, leading to hypotension, further decrease in organ perfusion, and even cardiac arrest. Ventilation with low tidal volumes (6 to 8 mL/kg) and moderate positive end-expiratory pressure (PEEP), producing low inspiratory alveolar or plateau pressures, appears to be the best pattern to prevent deterioration of hemodynamics and decrease the likelihood of ARDS.43 In intubated, spontaneously breathing patients, airway pressure release ventilation, in which spontaneous breathing is superimposed on mechanical ventilation by intermittent sudden, brief decrease of CPAP, provides improved ventilation/perfusion (V./Q.) matching and systemic blood pressure, lower sedation requirements, greater oxygen (O2) delivery, shorter periods of intubation, and a decreased incidence of ventilator-associated pneumonia, which occurs in up to 30% of ventilated patients with pulmonary contusion.41,44,45 Severe unilateral pulmonary contusion unresponsive to these measures may be treated by differential lung ventilation via a double-lumen endobronchial tube. In bilateral severe contusions with life-threatening hypoxemia, high-frequency jet ventilation may enhance oxygenation and cardiac function, which may be compromised by concomitant myocardial contusion or ischemia.46
obstruction, and significant pre-existing chronic pulmonary disease are indications for tracheal intubation and mechanical ventilation. Outcome in these patients may be dependent on the pattern of ventilation. In head injury patients, unless the clinical evidence suggests imminent cerebral herniation, hyperventilation must be avoided because it increases cerebral vasoconstriction, thus decreasing perfusion with accumulation of cerebral lactic acid immediately after its institution.42 In hypovolemic patients, hyperventilation may interfere with venous return and cardiac output, leading to hypotension, further decrease in organ perfusion, and even cardiac arrest. Ventilation with low tidal volumes (6 to 8 mL/kg) and moderate positive end-expiratory pressure (PEEP), producing low inspiratory alveolar or plateau pressures, appears to be the best pattern to prevent deterioration of hemodynamics and decrease the likelihood of ARDS.43 In intubated, spontaneously breathing patients, airway pressure release ventilation, in which spontaneous breathing is superimposed on mechanical ventilation by intermittent sudden, brief decrease of CPAP, provides improved ventilation/perfusion (V./Q.) matching and systemic blood pressure, lower sedation requirements, greater oxygen (O2) delivery, shorter periods of intubation, and a decreased incidence of ventilator-associated pneumonia, which occurs in up to 30% of ventilated patients with pulmonary contusion.41,44,45 Severe unilateral pulmonary contusion unresponsive to these measures may be treated by differential lung ventilation via a double-lumen endobronchial tube. In bilateral severe contusions with life-threatening hypoxemia, high-frequency jet ventilation may enhance oxygenation and cardiac function, which may be compromised by concomitant myocardial contusion or ischemia.46
Systemic air embolism occurs mainly after penetrating lung trauma and blast injuries, or less frequently after blunt thoracic trauma that produces lacerations of both distal air passages and pulmonary veins.47 Positive-pressure ventilation after tracheal intubation may then result in entrainment of air into the systemic circulation. Hemoptysis, circulatory instability, and CNS dysfunction immediately after starting artificial ventilation, as well as detection of air in blood from the radial artery, establishes the diagnosis. Air bubbles may also be seen in the coronary arteries during thoracotomy. Surgical management involves immediate thoracotomy and clamping of the hilum of the lacerated lung. Respiratory maneuvers that minimize or prevent air entry into the systemic circulation include isolating and collapsing the lacerated lung by means of a double-lumen tube or ventilation with the lowest possible tidal volumes via a single-lumen tube.47 Transesophageal echocardiography (TEE) of the left side of the heart may permits visualization of air bubbles and their disappearance with therapeutic maneuvers.
Management of Shock
In bleeding patients the primary goal is the urgent surgical control of the source of bleeding. Certain types of bleeding sources, however, may be temporarily controlled with nonsurgical measures, such as finger compression of open neck injuries and tourniquet control of external bleeding from extremities. Tourniquets should be removed as soon as urgent surgical control is achieved to avoid pressure-induced nerve damage, skin necrosis, or limb ischemia.
Evaluation of the severity of hemorrhagic shock in the initial phase is based on the mechanism and anatomical pattern of injury, hemodynamic data, and the response to fluid resuscitation. Free falls from heights >6 m, high-energy deceleration impact, and high velocity gunshot wounds are very likely to produce major damage and bleeding. Thoracoabdominal and pelvic injuries also are likely to be associated with major bleeding. Immediate evaluation of these anatomical sites with radiographs of the chest and pelvis, focused abdominal sonography for trauma (FAST), CT, or rarely, diagnostic peritoneal lavage (DPL), is necessary. Patients with significant intra-abdominal fluid recognized with these tests and hemodynamic instability require immediate surgical intervention. Those who are suspected to have occult abdominal bleeding based on a high-risk mechanism of injury but who are hemodynamically stable must undergo further evaluation with CT. The modern multislice CT devices available in most trauma centers can provide early whole-body scanning with or without contrast within a few minutes.48 By using contrast, a delayed-phase CT can also demonstrate the active bleeding site.
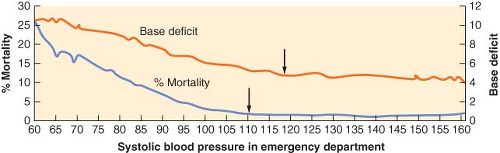
Clinical assessment using hemodynamic data is based on a few relatively insensitive and nonspecific clinical signs. For example, tachycardia, which is traditionally used as an index of hypovolemia, may be absent in up to 30% of hypotensive trauma patients because of increased vagal tone, chronic cocaine use, or other reasons.49 Inability of the patient to elevate the heart rate in the face of hypoperfusion is considered a predictor of increased mortality independent of severity of injury, systemic blood pressure, or the presence of a head injury.49 In contrast, by increasing catecholamine output, tissue injury and associated pain may maintain tachycardia and normal systemic blood pressure in the presence or absence of hypovolemia without necessarily increasing the cardiac index or tissue oxygen delivery. In fact, in this situation an increase in intestinal vascular resistance and a decrease in splanchnic blood flow may occur, and if prolonged, may allow entry of intestinal micro-organisms into the circulation and increase the likelihood of subsequent sepsis and organ failure.50,51,52 Thus, equating a normal heart rate and systemic blood pressure with normovolemia during initial resuscitation may lead to loss of valuable time for treating underlying occult hypovolemia or hypoperfusion. This is especially true in the elderly trauma population (age >65) in whom significant tissue hypoperfusion in the presence of normal blood pressure is more likely than in younger patients.53 Traditionally, the normal systolic blood pressure (SBP) is defined as 90 mm Hg. Recent findings suggest that trauma patients with this level or lower emergency department (ED) SBP have a higher mortality, higher blood lactate levels, and greater base deficits than civilian trauma patients with a SBP of 110 mm Hg and injured soldiers with a SBP of 100 mm Hg54,55 (Fig. 52-4). Thus, the optimal SBP in the trauma patient appears to be 100 to 110 mm Hg. Although traditional vital signs are relatively unreliable for recognizing life-threatening shock, heart rate, systemic blood pressure, pulse pressure, respiratory rate, urine output, and mental status are still used as early clinical indicators of the severity of hemorrhagic shock. (Table 52-3).50,56 Shock index (SI), a value derived by dividing the heart rate by the SBP, appears to be a more accurate indicator of early hemorrhagic shock and a predictor of mortality than the individual vital signs. In normal individuals, SI varies between 0.58 and 0.64 (mean 0.61) increasing from 0.70 to 0.80 (mean 0.75) after a moderate degree of blood loss. In the elderly, it has been demonstrated that age times SI identifies early shock and predicts mortality better than SI itself.57
Table 52-2. Guidelines for Management of Traumatic Shock | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Table 52-3. Advanced Trauma Life Support Classification of Hemorrhagic Shocka | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Finally, the response to initial fluid resuscitation in the form of lactated Ringer’s or normal saline solution of about 2 L, or 20 mL/kg in children, over a period of 15 to 30 minutes may allow estimation of the severity of hemorrhage (Table 52-4).45 The goal of therapy is to re-establish organ perfusion. Overinfusing fluids in patients who have normalized their SBP may lead to further bleeding by increasing arterial and venous pressures, diluting clotting factors and platelets, reducing body temperature, and decreasing blood viscosity.58,59 Bickell et al.60 showed that delaying fluid resuscitation until surgical control of bleeding in victims of penetrating trauma improved survival to hospital discharge and decreased the length of hospital stay. Although many experimental studies have confirmed the findings of Bickell et al.,60 it has also become clear that withholding fluids completely can result in as much harm as vigorous resuscitation.61 In contrast, slow infusion of isotonic or hypertonic crystalloids and preferably of packed red blood cells (PRBCs), titrated to slightly lower than normal systemic pressure, had beneficial effects on animal survival without tissue injury or organ failure. A clinical study conducted subsequent to Bickell et al.60 on hypotensive resuscitation demonstrated better outcome in penetrating, but not in blunt trauma.62 Nevertheless, while this practice is contraindicated in traumatic brain and spinal cord injuries and in elderly patients with chronic systemic hypertension where adequate perfusion is crucial,59 it emphasizes the useful fact that fluid administration in excess of that needed to achieve normovolemia prior to control of hemorrhage may be deleterious.58 Early use of vasopressors to maintain hemodynamic stability has also been shown to be associated with deleterious effects.63 However, judicious use of these drugs along with carefully titrated fluids may offer some advantages.
Crystalloids are used in the vast majority of trauma centers for initial resuscitation.59 Hypertonic saline (250 mL 7.5% with 6% dextran-70) is safe and may be associated with lower intracranial pressure than normal saline in brain-injured patients59 and may improve hemodynamics in hypotensive penetrating trauma patients.64 Although colloid solutions are associated with an increased risk of organ failure and death in trauma victims, a meta-analysis showed no difference in mortality between colloids and crystalloids.65 If used, hydroxyethyl starch solutions (maximum 20 mL/kg) should probably be given priority over albumin solutions because the possible deleterious effects of colloids have mostly been associated with albumin.
Some of the proven markers of organ perfusion can be used during early management to set the goals of resuscitation. Of
these, the base deficit and blood lactate level are the most useful and practical tools during all phases of shock, including the earliest. The base deficit reflects the severity of shock, the oxygen debt, changes in O2 delivery, the adequacy of fluid resuscitation, and the likelihood of multiple organ failure and survival with reasonable accuracy in previously healthy adult and pediatric trauma patients.59 Base deficit is considered a better prognostic marker than the arterial pH. A base deficit between −2 and −5 mmol/L suggests mild shock, between −6 and −9 mmol/L indicates moderate shock, whereas ≤-10 mmol/L is a sign of severe shock.59 An admission base deficit below −5 to −8 mmol/L correlates with increased mortality. Thus, normalization of the base deficit is one of the end points of resuscitation. Elevation of the blood lactate level is less specific than base deficit as a marker of tissue hypoxia because it can be generated in well-oxygenated tissues by increased epinephrine-induced skeletal muscle glycolysis, accelerated pyruvate oxidation, decreased hepatic clearance of lactate, and early mitochondrial dysfunction. All these conditions may be present in the trauma patient. Thus, blood lactate and base deficit may not closely correlate with each other. Nevertheless, in most trauma victims an elevated lactate level correlates with other signs of hypoperfusion, rendering it an important marker of dysoxia and an end point of resuscitation. The normal plasma lactate concentration is 0.5 to 1.5 mmol/L; levels >5 mmol/L indicate significant lactic acidosis. The half-life of lactate is approximately 3 hours; thus, the level decreases rather gradually after correction of the cause. Failure to clear lactate within 24 hours after reversal of circulatory shock is a predictor of increased mortality.59
these, the base deficit and blood lactate level are the most useful and practical tools during all phases of shock, including the earliest. The base deficit reflects the severity of shock, the oxygen debt, changes in O2 delivery, the adequacy of fluid resuscitation, and the likelihood of multiple organ failure and survival with reasonable accuracy in previously healthy adult and pediatric trauma patients.59 Base deficit is considered a better prognostic marker than the arterial pH. A base deficit between −2 and −5 mmol/L suggests mild shock, between −6 and −9 mmol/L indicates moderate shock, whereas ≤-10 mmol/L is a sign of severe shock.59 An admission base deficit below −5 to −8 mmol/L correlates with increased mortality. Thus, normalization of the base deficit is one of the end points of resuscitation. Elevation of the blood lactate level is less specific than base deficit as a marker of tissue hypoxia because it can be generated in well-oxygenated tissues by increased epinephrine-induced skeletal muscle glycolysis, accelerated pyruvate oxidation, decreased hepatic clearance of lactate, and early mitochondrial dysfunction. All these conditions may be present in the trauma patient. Thus, blood lactate and base deficit may not closely correlate with each other. Nevertheless, in most trauma victims an elevated lactate level correlates with other signs of hypoperfusion, rendering it an important marker of dysoxia and an end point of resuscitation. The normal plasma lactate concentration is 0.5 to 1.5 mmol/L; levels >5 mmol/L indicate significant lactic acidosis. The half-life of lactate is approximately 3 hours; thus, the level decreases rather gradually after correction of the cause. Failure to clear lactate within 24 hours after reversal of circulatory shock is a predictor of increased mortality.59
Table 52-4. Response to Initial Fluid Resuscitationa | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Measurement of hemoglobin (Hgb) or hematocrit (Hct) helps in managing the bleeding trauma patient. However, decision making based on a single Hct value may lead to erroneous management decisions. Trauma patients, if not treated with adequate crystalloids or colloids or those receiving PRBC transfusion, can maintain a normal Hct despite ongoing bleeding and delay in surgical control. On the other hand, serial Hct measurements and consideration of the type and amount of fluid received may be useful in deciding the timing and amount of transfusion.66 The usefulness of Hgb or Hct as a PRBC transfusion threshold remains unclear, although the recommended target Hgb concentration in all phases of management is 7 to 9 g/dL, including brain-injured patients in whom tissue oxygenation is most relevant.59 In brain-injured patients, although several studies demonstrated that increasing the Hgb level to 9 or 10 g/dL from lower levels with PRBCs of <19 days storage increased brain oxygenation in 75% of patients,67,68 data from other sources showed either increased morbidity and mortality with PRBC transfusion69 or improved neurological outcome when the Hct value was kept <30 (Hgb <10 g/dL) for longer periods.70 Transfusion of PRBC has been shown to be an independent risk factor for mortality, lung injury, increased infection rate, renal failure, and intensive care unit (ICU) and hospital length of stay in trauma patients, especially when the transfused red cells are older than 14 days; this finding was true independent of the severity of shock.71,72 Nevertheless, this concern should not preclude timely and adequate administration of blood products.
Normally, type-specific crossmatched blood can be available in most centers in about 30 minutes, including transport time. Type-specific uncrossmatched blood can be available in even less time for patients with severe hemorrhage. However, if the situation dictates immediate transfusion, type O, Rh-positive, AB-negative fresh frozen plasma (FFP) is satisfactory in most situations. Controversy exists about the use of uncrossmatched type O PRBC because of concern about the development of alloantibodies and allergic reactions. Dutton et al.,73 reviewing their experience in 161 patients receiving 581 units of universal donor blood, demonstrated that only 1 of the 10 Rh-negative males receiving O, Rh-positive blood developed alloantibodies. All four females in the series received type O, Rh-negative blood without apparent problem.
Liquid plasma differs from FFP in that it is frozen at −180°C within 8 to 24 hours, whereas FFP is frozen within 8 hours. It contains all of the stable proteins found in FFP, although in slightly lower concentrations. The major difference is a 25% reduction of factor VIII. One unit of FFP contains approximately 7% of the
coagulation factor activity of a 70-kg man. Thawing of FFP or liquid plasma takes about 30 minutes. The recommended ratio of FFP and platelets to PRBCs varies widely, ranging from 1:10 to 2:3 for FFP to PRBCs and from 6:10 to 12:10 for platelets to PRBCs.77 Nevertheless, military data demonstrate that the death rate was 65% when the plasma to PRBC ratio was 1:8, 34% when it was 1:2.5, and 19% for 1:1.4.78
coagulation factor activity of a 70-kg man. Thawing of FFP or liquid plasma takes about 30 minutes. The recommended ratio of FFP and platelets to PRBCs varies widely, ranging from 1:10 to 2:3 for FFP to PRBCs and from 6:10 to 12:10 for platelets to PRBCs.77 Nevertheless, military data demonstrate that the death rate was 65% when the plasma to PRBC ratio was 1:8, 34% when it was 1:2.5, and 19% for 1:1.4.78
Table 52-5. Massive Transfusion Protocol Used in Grady Memorial Hospital in Atlanta | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
Currently many trauma centers use hemostatic resuscitation (massive transfusion) protocols during initial resuscitation of major traumatic hemorrhage in the ED and OR. These involve administering a relatively limited quantity of crystalloid solutions and volume replacement with liquid plasma or FFP and PRBCs. In addition, platelets and cryoprecipitate are given regularly. One such protocol, used in Grady Memorial Hospital in Atlanta, Georgia, is shown in Table 52-5.79
Timely initiation of massive transfusion or hemostatic resuscitation protocol is associated with improved survival and reduced transfusion.80,81 However, identification of these patients early enough is difficult. Much effort has been directed recently at specifying criteria to identify these patients. Cotton et al.82 recently validated previously proposed simple criteria as an early massive transfusion trigger. The ABC score is calculated by assigning a value of 0 or 1 to each of four parameters: penetrating mechanism, positive FAST, arrival SBP <90 mm Hg, and heart rate >120 beats per minute. A score of ≥2 was associated with an increased likelihood of massive transfusion. Larson et al.81 demonstrated that the presence of any two of the of four admission findings (heart rate >110 beats per minute, SBP <110 mm Hg, base deficit <−6, and Hgb <11) predicted massive transfusion with high likelihood. Finally Callcut et al.83 demonstrated that each of the five massive transfusion trigger variables proposed by the military did not have the same predictive ability. Of SBP <90 mm Hg, Hgb <11 g/dL, body temperature <35.5°C, International Normalized Ratio (INR) >1.5, and base deficit <−6, INR was the most predictive of massive transfusion. However, generally the presence of three or more of these five triggers suggests a greater likelihood for the need of massive transfusion. Finally, rapid thromboelastography (r-TEG) can be useful to decide on early (<1 hour) administration of FFP and platelets.84
Rapid establishment of venous access with large-bore cannulae placed in peripheral veins that drain both above and below the diaphragm is essential for adequate fluid resuscitation in the patient who is severely injured. When vascular collapse and extremity injury impair access to arm or leg vessels, percutaneous cannulation of the internal jugular, subclavian, or femoral veins can be performed. Ultrasound guidance may facilitate cannulation of the internal jugular vein and prevent needle entry and infusion of fluids into the pleural space in patients with a large hemothorax. Ultrasound may also be used for infraclavicular access to the axillary vein, the cephalic or basilic veins at the midarm level, or to the femoral vein. If necessary, a cutdown to a saphenous or arm vein can be rapidly performed in older children and adults. In children <5 years of age, intraosseous cannulation has a high success rate and a low incidence of complications. Infusion rates comparable with those obtained with intravenous lines are possible in small children, although a pressure infusion device may be necessary to achieve adequate flow.
Patients who arrive in the ED in cardiac arrest require advanced cardiac life support. However, the success rate of external cardiac massage in hypovolemic trauma victims is likely to be low.85 ED thoracotomy not only permits performance of open cardiac massage, but also aids resuscitation efforts by allowing drainage of pericardial blood, control of cardiac and great vessel bleeding, application of a cross-clamp to the aorta, and rapid administration of fluids through a small Foley catheter introduced into the right atrium, or in desperate situations, through a large-bore catheter or introducer in the descending aorta. This procedure is not indicated in blunt torso trauma. The mortality rate is similar regardless of whether it is attempted or not.86 In penetrating injuries, depending on the presenting condition of the patient, the initial success rate may be as high as 70%, but the neurologically intact hospital discharge rate is only 10% to 15%.86,87
Early Management of Specific Injuries
Head Injury
Approximately 40% of deaths from trauma are caused by head injury, and indeed, even a moderate brain injury may increase the mortality rate of patients with other injuries. In nonsurvivors, progression of the damaged area beyond the directly injured region (secondary brain injury) can be demonstrated at autopsy.88 The major factor in secondary injury is tissue hypoxia, which results in lactic acidosis, free radical generation, prostaglandin synthesis and release of excitatory amino acids (primarily glutamate), lipid peroxidation and breakdown of cell membranes, entry of large
quantities of sodium, calcium, and water into the cells, and leakage of fluid from the blood vessels into the extracellular space.89,90 This process results in brain edema and both regional and global disturbances of the cerebral circulation. Thus, of all the possible secondary insults to the injured brain, decreased oxygen delivery as a result of hypotension and hypoxia has the greatest detrimental impact (Table 52-6).91,92
quantities of sodium, calcium, and water into the cells, and leakage of fluid from the blood vessels into the extracellular space.89,90 This process results in brain edema and both regional and global disturbances of the cerebral circulation. Thus, of all the possible secondary insults to the injured brain, decreased oxygen delivery as a result of hypotension and hypoxia has the greatest detrimental impact (Table 52-6).91,92
Table 52-6. Effects on Outcome of Secondary Insults Occurring from Time of Injury Through Resuscitationa | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Brain injury by itself does not cause hypotension in adults except as a preterminal event. However, more than half of the patients with severe head trauma have other injuries that render approximately 15% of them hypotensive. Approximately 30% are hypoxic on admission as a result of central respiratory depression or associated chest injuries. Furthermore, exposure to these insults is likely to occur during any phase of the continuum of hospital care: in the radiology unit, the OR, the postanesthesia care unit, the ICU, or elsewhere. The most common early complications of head trauma are intracranial hypertension, brain herniation, seizures, neurogenic pulmonary edema, cardiac dysrhythmias, bradycardia, systemic hypertension, and coagulopathy.
Diagnosis
Mental impairment after trauma may have any of several etiologies. However, the possibility of hypoxia and shock must always be considered first. If consciousness remains depressed despite ventilation and fluid replacement, a head injury is assumed to be present and the patient is managed accordingly. As noted, hypotension is the most important cause of death in the head-injured patient. Chesnut92 demonstrated that a single episode of SBP <90 mm Hg is associated with a 50% increase in mortality, and subsequent episodes or lower pressures increase mortality even further.93 Therefore, every effort should be made to support the blood pressure with fluids and vasopressors (preferably phenylephrine, which does not constrict cerebral vessels) and ensure adequate oxygenation before the unconscious patient is evaluated. A baseline neurologic examination should be performed after initial resuscitation but before any sedative or muscle relaxant agents are administered, and this should be repeated at frequent intervals because the patient’s condition may change rapidly. Anesthetic and adjunct drugs may render an adequate neurologic examination impossible; thus, long-acting muscle relaxants, opioids, sedatives, or hypnotics should be given selectively.91,94
Consciousness can be initially assessed within a few seconds using the AVPU system (alert; responds to verbal stimuli; responds to pain; unresponsive) (Table 52-7). More precise information is provided by the GCS (Table 52-7), which provides a standard means of evaluating the patient’s neurologic status. In this test, the sum of the scores obtained for eye opening, verbal response, and
motor activity correlates with the state of consciousness, the severity of the head injury, and the prognosis.94 Assessment of motor function should be performed on the extremity that responds best. The limb affected by neurologic injury is examined, but the result is not considered in the GCS.
motor activity correlates with the state of consciousness, the severity of the head injury, and the prognosis.94 Assessment of motor function should be performed on the extremity that responds best. The limb affected by neurologic injury is examined, but the result is not considered in the GCS.
Table 52-7. Two-Level Initial Evaluation of Consciousness | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Dilatation and sluggish response of the pupil is a sign of compression of the oculomotor nerve by the medial portion of the temporal lobe (uncus). A maximally dilated and unresponsive “blown” pupil suggests uncal herniation under the falx cerebri. The presence of similar findings in ocular injuries makes interpretation of pupillary findings difficult when eye and head injuries coexist. However, the pupillary reaction to light is usually more sluggish in the head-injured patient.
CT scanning is used for the diagnosis of most acute head injuries. Positive CT findings after acute head injury include midline shift, distortion of the ventricles and cisterns, effacement of the sulci in the uninjured hemisphere, and the presence of a hematoma at any location in the cranial vault. Subdural hematomas usually have a concave border, whereas epidural hematomas present with a convex outline classically termed a lenticular configuration. Patients in severe coma (GCS <8) have a 40% likelihood of an intracranial hematoma. Those with higher GCS scores are less likely to have had intracranial bleeding although it is evident that the significant incidence of this complication even in these patients necessitates a CT study, preferably with contrast enhancement. Other benefits of CT scanning include detection of intracranial air and depressed skull fractures.
Management
Normalization of the ICP has been shown to reduce mortality.100 Effective reduction in ICP can be provided, or at least aided, by administration of mannitol, an important part of the management of severe head injury. It is administered in boluses of 0.25 to 0.5 g/kg and repeated every 4 to 6 hours as needed to control the ICP.91 Higher doses, up to 2 g/kg, are recommended by some authors.101 In addition to its osmotic diuretic effect, this agent may improve cerebral blood flow (CBF) and O2 delivery by reducing the hematocrit and thus the blood viscosity, improving CBF and oxygen delivery.91 There is a risk of hypovolemia and resultant hypotension when therapeutic doses of mannitol are used. If the ICP elevation persists, additional doses of mannitol should be given cautiously. Acute mannitol toxicity, manifested by hyponatremia, high serum osmolality, and a gap between calculated and measured serum osmolality >10 mOsm/L, may result when the drug is given in large doses (2 to 3 g/kg) or to patients with renal failure. Mannitol should be used with great care in the presence of hypotension, sepsis, nephrotoxic drugs, or pre-existing renal disease, because these may also precipitate renal failure.91 Further, the effects of mannitol result from its activity in regions of the brain where the blood–brain barrier is intact. It may exacerbate edema in injured areas in which it may easily enter the tissues.
The addition of relatively small volumes of hypertonic saline in concentrations between 3% (6 to 8 mL/kg) and 7.5% (4 mL/kg) followed by infusion of LR may be beneficial in multiple trauma patients with head injury.102 In addition, hypertonic saline (15% solution), in bolus doses of 0.42 mL/kg, is as efficacious as mannitol for initial therapy of elevated ICP in this patient population.101 Like mannitol, hypertonic saline draws fluid from the intracellular space and, thus, in addition to restoring the blood volume, it reduces brain edema and prevents elevation of the ICP.103 On the other hand, hypertonic saline may, also like mannitol, increase edema in the injured region of the brain.104 The intravascular volume expansion produced by hypertonic saline is transient. It can be prolonged by addition of 6% dextran-70 or hetastarch to the solution. However, administration of hypertonic saline cannot be maintained for long periods. It may cause hypernatremia, hyperosmolality, or hyperchloremic acidosis, probably from renal bicarbonate loss secondary to increased levels of chloride (Cl−). Serum concentrations of sodium (Na+) and Cl− and the patient’s acid–base status should be followed, and the administration of hypertonic saline should be discontinued if plasma Na+ reaches 160 mEq/L. Because of these considerations, and the fact that there has been no standardization of the concentration, the dose, or the duration of treatment, the use of hypertonic saline should still be considered experimental therapy.91,105 Resuscitation with colloid solutions (hetastarch, pentastarch, pentafraction, human albumin 5% and
25%, or dextran) provides a sustained improvement in vital signs, but the increase in colloid osmotic pressure produced by these solutions may not have an important role in reducing brain edema.
25%, or dextran) provides a sustained improvement in vital signs, but the increase in colloid osmotic pressure produced by these solutions may not have an important role in reducing brain edema.
Hyponatremia in these patients results from intravascular volume expansion rather than sodium loss; thus treatment with saline solutions is not appropriate. Because of a synergistic action between mannitol and loop diuretics in improving the ICP, addition of furosemide may be a safer and more effective treatment than increasing the dose of mannitol when intracranial hypertension persists. Until about 1995, hyperventilation to a PaCO2 of 25 to 30 mm Hg was a mainstay of the therapy of head injury. However, brain ischemia, which is probably the most threatening consequence of head injury, is likely to occur during the first 6 hours after trauma even when the CPP is maintained above the generally recommended 50 to 70 mm Hg.106 This hypoperfusion seems to be caused largely by increased cerebral vascular resistance, which may be enhanced by hyperventilation. However, some degree of hyperventilation may be necessary for short periods of time in patients who have severe injuries and elevated ICP that does not respond to normal ventilation and diuretics, although this should not be used during the first 24 hours following injury.91 Its use after the initial phase should be based on monitoring of the ICP and, if available, the jugular venous oxygen saturation (SjvO2) and arteriojugular venous difference of oxygen (AVDO2). It should be noted that hyperventilation in the severely brain-injured patient may also be associated with acute lung injury.107
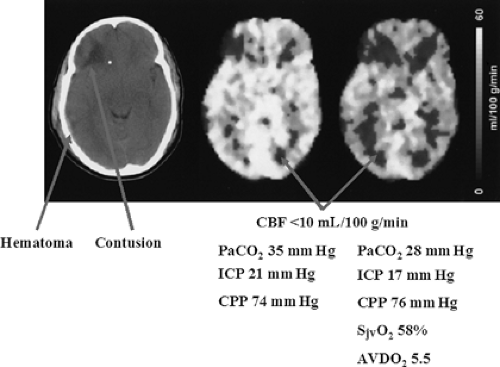
Measurement of the SjvO2 is used in some centers as a guide to therapy of the head-injured patient.108 A catheter is passed retrograde into the jugular bulb under fluoroscopic control. The O2 saturation may be measured with a co-oximeter or continuously by means of a fiberoptic sensor.108 An SjvO2 of <50% is considered critical desaturation. The AVDO2 is a standard measure of the brain’s oxygen supply to demand ratio. It is equal to 1.34 × Hgb × (SaO2 – SjvO2) (the saturations are expressed as decimal values), and normally is approximately 6. An increase in this value is a sign of insufficient blood flow, whereas a subnormal level indicates hyperemia. A reduction in ICP with elevation of CPP during treatment is reflected by a rise in SjvO2 and a narrowing of the AVDO2, presumably reflecting an improvement in the circulation to the brain. Unfortunately, several shortcomings of the technique have hindered its universal acceptance. Because all of the cerebral veins drain into the cavernous sinus and from there into the jugular bulbs, AVDO2 measures only global O2 consumption, which may well be very different from the situation in the injured region. Indeed, Coles106 has demonstrated by positron emission tomography (PET) scanning that a significant increase in the region of critical hypoperfusion resulting from hyperventilation was not necessarily associated with a correspondingly abnormal SjvO2 or AVDO2 (Fig. 52-6). Patient or catheter movement may also alter the measured jugular bulb venous oxygen tension. Thus, there may be a high proportion of inaccurate values—as high as nearly two-thirds—although recent advances in the technique have probably reduced these errors. Cruz109 has suggested that jugular venous monitoring should be used only in sedated, paralyzed patients.
If the ICP remains elevated despite all of these measures, pentobarbital (3 to 10 mg/kg given over 0.5 to 2.5 hours, followed by a maintenance infusion of 0.5 to 3.0 mg/kg/hr, aimed at a serum concentration between 2.5 and 4.0 mg/dL) may be required. High-dose barbiturates are of no value in the routine therapy of head injury and should be used only for refractory ICP elevation. Of course, immediate surgical decompression, especially of epidural hematomas, is an important factor in reducing morbidity and mortality.
Over the past decade there has been much debate regarding optimal blood glucose in critically ill patients. Brain-injured patients are unique members of this group because brain metabolism is altered by the injury and is heavily dependent on glucose. Hypoglycemia (<40 gm/dL), which may cause metabolic crisis, and hyperglycemia (>200 gm/dL) can cause detrimental effects through excitotoxicity, oxidative stress, and inflammatory cytokine release. Tight insulin control therapy (80 to 110 mg/dL) has been associated with episodes of hypoglycemia. As a result, the current recommendations are to maintain glucose levels of 110 to 180 mg/dL.110
Nearly 75% of severely brain-injured patients expire within the first 3 days from the initial trauma. Many of the survivors will later succumb to non-neurologic organ dysfunction involving pulmonary failure and cardiac impairment, which may be related to sympathetic hyperactivity. β-blocker therapy has been proposed as a treatment that may be beneficial in these patients.111 The optimal agent, the dose level, the timing, and the duration of treatment, however, remain to be determined.
If the patient is hemodynamically stable, a CT scan is performed. The strictest attention should be paid to ensure adequate oxygenation, ventilation, blood pressure, and ICP control during the procedure. If the patient is hemodynamically unstable or requires emergency surgery for associated injuries and has a history suggesting a head injury, even though a significant intracranial hematoma is unlikely on clinical grounds, intraoperative ICP monitoring is indicated to permit rapid detection of ICP elevation. Both intracranial hematomas and hemorrhage in other regions have a high surgical priority. In the multiple trauma victim, prioritization between the two is based on the severity of each injury. Because there is no time to obtain a CT scan of the head in patients with both profuse hemorrhage and brain herniation, the patient is brought directly to the OR for simultaneous control of the bleeding site and evacuation of the intracranial hematoma.
The site of the craniotomy can be determined by a ventriculogram or an ultrasound examination with a pencil-tip probe; both tests may be performed under local anesthesia through a frontal burr hole.
The site of the craniotomy can be determined by a ventriculogram or an ultrasound examination with a pencil-tip probe; both tests may be performed under local anesthesia through a frontal burr hole.
Table 52-8. Six-Month Outcomes for Patients with Brain Injury in Various Studies | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree