Transplant Anesthesia
Marie Csete
Dalia Banks
Gerard Manecke
Kathryn Glas
Key Points
Related Matter
Left Liver Lobe Donation
Transplantation is the standard of care for many end-stage diseases. About 113,000 patients are on solid-organ transplant waiting lists in the United States. Organ donation has not kept pace with demand, resulting in many practice changes to increase the donor pool, including more use of living-related organ donation, donor organs once considered marginal (extended criteria donors [ECDs]), grafts from “donation after cardiac death” (DCD) donors, as well as paired kidney donations (kidney swaps). All of these changes impact anesthetic management of organ recipients.
The United Network of Organ Sharing (UNOS; www.unos.org) was created by the 1984 National Organ Transplant Act to manage the organ procurement and transplant network for efficient and equitable distribution of donated organs. The system for organ placement received a technology upgrade in 2006 with the launch of DonorNet, an electronic resource for matching and distribution of organs around the United States. Speed of placement is important, as brain-dead donors are unstable, and ECD grafts are particularly important to transplant with minimal cold ischemic times. In general, maximum cold ischemic times are 4 to 6 hours for heart or lung grafts, 12 to 24 hours for livers, and up to 72 hours for kidneys. The UNOS website data contain center- and region-specific transplant databases, regularly updated.
The Scientific Registry of Transplant Recipients (www.ustransplant.org) is a source of transplant data important to clinicians, patients, and researchers. US centers performed 27,527 transplants in 2009, and almost 200,000 Americans are living with transplanted organs. Data on death rates while on transplant waiting lists are sobering, pointing to the complexity of illness in these patients and the need for more organ donation. In 2008, the rate of death on the waiting list for hearts was 17%, for kidneys 6.3%, and for livers 11.4%. In 2010, in the United States (including Washington, D.C., and Puerto Rico), 28,663 transplants were performed: 16,899 kidneys, 350 pancreata, 828 kidneys/pancreata, 6,291 livers, 2,333 hearts, 1,770 lungs, 41 hearts/lungs, and 151 intestinal transplants. In major transplant centers, specialist anesthesiology teams participate in the preoperative assessment and optimization of patients for major organ transplant procedures. Directors of liver transplant anesthesiology teams are now required to complete transplant continuing medical education. Solid-organ transplantation is also a critical component of many anesthesia training programs.
Anesthetic Management of Organ Donors
Brain-dead Donors
Brain death is associated with hemodynamic instability, wide swings in hormone levels, systemic inflammation, and oxidant stress, all of which negatively impact donor organ function.3 Just after brain death, adrenergic surges can cause ischemia and ischemia–reperfusion injuries. Studies of head trauma patients suggest that the onset of brain death is associated with a transient period of hypotension with increased cardiac index and tissue perfusion. During this period, vasoactive drugs administered to increase blood pressure can cause rapid circulatory deterioration.4 This period precedes the autonomic storm associated with herniation of the brain and emphasizes the wide dynamic swings in blood chemistries and hemodynamics after brain death. The timing of therapies to support hemodynamics is difficult as catecholamine storm is often followed quickly by pituitary failure. Once pituitary failure ensues, hormone therapy may help stabilize donors hemodynamically and thereby extend the donor pool.5,6,7,8 However, with the inherent difficulty of controlling studies in brain-dead patients, proof that routine donor hormone therapy is beneficial is difficult to obtain. A standard hormone therapy regimen is triiodothyronine (4-μg intravenous [IV] bolus, then 3 μg/hr); methylprednisolone, 15 mg/kg intravenously every 24 hours; desmopressin, 1 U then 0.5 to 4 U/hr to maintain systemic vascular resistance (SVR) at 800 to 1,200 dyne/s/cm5 (and reduce the polyuria of diabetes insipidus). Insulin infusion to maintain blood glucose 120 to 180 mg/dL is also recommended, and recent studies support glucose control for maintaining donor kidney graft quality.9 Evidence that desmopressin is better than norepinephrine for maintaining donor heart quality is solid, evidence for triiodothyronine is not strong.10 Other medications that should be available for the donor operation are broad-spectrum antibiotics, mannitol and loop diuretics, and heparin.
Because donor yield for hearts is only 42%, consensus conferences have been used to improve evaluation and utilization of cardiac donor organs.11 Donor heart history is very important, and electrocardiography and echocardiography are mandatory. The ideal heart donor is <50 years old and is hemodynamically stable. Presence of major chest trauma, cardiac disease, active infection, prolonged cardiac arrest, malignancy, human immunodeficiency virus or hepatitis, or intracardiac injections moves the donor from ideal to marginal status. Overall health status of the donor prior to determination of brain death can facilitate a directed laboratory evaluation. Cardiac catheterization is often requested for male donors >45 years old, for females >50 years old, or for young donors with significant personal or family history of coronary artery disease. For recipients with pulmonary hypertension, younger donors, short ischemic time, low donor inotrope requirement, and oversized organs are preferred. Human leukocyte antigen (HLA) typing and ABO blood group compatibility are performed. The donor heart size should be within 20% to 30% of the recipient’s heart size.
Anesthetic management during organ harvest is guided by the needs of the procurement teams, who may come from several centers and have discrepant requests, depending on the organs procured. UNOS has created a resource for managing organ donors in an effort to improve donor care and, therefore, the function of donated organs.a Transport of ventilated donors to the operating room (OR) often requires positive end-expiratory pressure (PEEP) valves attached to the Ambu bag to maintain
oxygenation. Recently, in a rare prospective trial, a lung-sparing ventilation strategy was shown to increase the lung donor pool. Patients ventilated with tidal volumes of 6 to 8 mL/kg body weight and 8 to 10 cm water (H2O) PEEP were more likely to be lung donors than patients managed with tidal volumes 10 to 15 mL/kg and 3 to 5 cm PEEP.12
oxygenation. Recently, in a rare prospective trial, a lung-sparing ventilation strategy was shown to increase the lung donor pool. Patients ventilated with tidal volumes of 6 to 8 mL/kg body weight and 8 to 10 cm water (H2O) PEEP were more likely to be lung donors than patients managed with tidal volumes 10 to 15 mL/kg and 3 to 5 cm PEEP.12
Generally, packed cells are used to maintain hematocrit of 30%, and fresh-frozen plasma (FFP) is used to maintain the international normalized ratio (INR) <1.5, although these practices are not evidence based. Surgeons procuring the lungs will want to keep the CVP low, and diuretics may be requested just prior to collection of the lungs. Surgeons procuring kidneys usually want high filling pressures. Donor oxygenation, perfusion, and normothermia are all important anesthetic goals, and the precise end points of therapy require coordination and communication with the various surgical teams. Generally, arterial PCO2 is maintained at 30 to 35 mm Hg.
Prior to lung removal, surgeons will perform bronchoscopy. An adapter, such as the Portex fiberoptic bronchoscopic swivel adapter (SIMS Portex, Inc., Keene, New Hampshire), facilitates ventilation during the procedure. Glucocorticoids may be requested, and on occasion, prostaglandin E1 is requested to improve circulation of the lung preservation solution. Surgical techniques have been developed to allow three recipients from one thoracic donor: two single-lung transplants and a heart transplant.16 The heart is removed first, leaving a small cuff of left atrium attached to the lungs. The harvesting team will ask for systemic heparinization just prior to exsanguination and excision. Cardioplegia is administered, the heart stops ejecting, and the heart is removed. The trachea is transected and the lungs are removed en bloc for later separation.
Donor lungs are more susceptible to injury in brain-dead patients before procurement than are other organs, likely from contusion, aspiration, or edema with fluid resuscitation. Consequently, many multiorgan donors do not meet the current strict criteria for lung donors. These criteria are listed in Table 51-1. The Pulmonary Council of the International Society for Heart and Lung Transplantation reviews evidence (or lack thereof) for these criteria.17 Lungs once considered marginal are being used increasingly because of this severe shortage of donor lungs. Exclusion of donors based on arterial blood gas or chest roentgenogram (CXR) (Table 51-1) is based on relatively small trials.18 With experience, lung transplantation using donors outside these boundaries does not negatively impact the recipient, and most centers rely on bronchoscopy to determine lung suitability for transplantation.19 Sputum Gram stains and cultures are routinely obtained on all lung donors. A positive Gram stain does not seem to impact outcome; however, organisms on bronchoalveolar lavage are associated with decreased survival.20 One study suggested that evidence of aspiration seen on bronchoscopy, bilateral pulmonary infiltrates, or persistent purulent secretions is a criterion for donor exclusion.21 Advanced donor age (>55 years) together with long ischemic time (>6 hours) are associated with poor transplant outcomes. In addition to the usual hematologic criteria, donor–recipient compatibility is based on height and/or total lung capacity.
Table 51-1. Ideal Deceased Lung Donor | ||
|---|---|---|
|
For heart retrieval, the surgeons perform a pericardiotomy, and the aortic root is cannulated for infusion of cardioplegia solutions. Following ligation of the great veins, the heart is compressed and exsanguinated, cardioplegia is given to induce cardiac arrest, and the aorta is cross-clamped. After cardiectomy the donor heart is preserved in cold ice slush. For donors who provide both lungs and the heart to a single recipient, a combined cardiopulmonary surgical extraction is performed.
A shortage of available donors has led to increased use of marginal donors and separate alternate transplant lists of recipients who consent to accept marginal donors. Increased risk of primary graft dysfunction is the main reason to avoid marginal donors. Marginal donors are typically used for patients who do not meet the standard recipient criteria, with advanced age a common reason for alternative listing. Common donor factors that lead to marginal status are abnormal hepatitis screening tests, left ventricular dysfunction or coronary artery disease, advanced age, and DCD.
Donation After Cardiac Death
develop its own protocols reflecting local constraints. Optimally, end-of-life care, including analgesia, is provided by the same medical team responsible for the care of the patient in the intensive care unit (ICU). Anesthesiologists do not necessarily have to be involved in DCD donor management, even when withdrawal of care occurs in the OR.24 The main principles for institutional guidelines are outlined in the ASA report and include the following: The decision to withdraw care must be made prior to and independent of any discussion about organ donation. Suitable DCD donors are those in whom death is anticipated within 1 to 2 hours of withdrawal of life support. With experience, DCD donors with longer time to cardiorespiratory arrest can be used successfully for kidney transplantation.25
Detailed protocols for interactions with family members of the donor, transplant teams, organ procurement organizations (OPOs), and OR personnel must be established as part of DCD planning. Informed consent is required for organ donation and for any preorgan recovery procedures, such as drug administration or vascular cannulation. A plan for the donor’s care should be in place if the patient does not die within the anticipated time frame, and ideally care should be transferred back to the team that knows the patient and family. Once the family decision to consent to DCD is made, the OPO can help guide management of the patient while the allocation of organs is being arranged. Uncontrolled, unanticipated crises that force the conversation about consent under extreme circumstances are obviously more difficult and may mean that the OPO cannot be involved in donor management because of time constraints. Nonetheless, uncontrolled DCD donors can be used for transplantation.26,27
Circulation and respiration must be absent for a minimum of 2 minutes before the start of organ recovery. Organ recovery started >5 minutes after respiratory and circulatory arrest may compromise donor organ quality, but this limit has now been extended with reasonable transplant outcomes. Predicting which patients will expire in 1 to 2 hours cannot be done with certainty. The most experienced program with DCD, University of Wisconsin, developed an evaluation tool to help predict which patients would die within 1 to 2 hours of withdrawal of support (Table 51-2).28
DCD donors provided 1,306 transplanted kidneys in 2008 (an increase of 12% over 2007), but 20.9% of DCD kidneys were discarded. DCD donors provided 276 livers (down from 2007), but 32.3% of the livers had to be discarded. Over half the pancreata of DCD donors had to be discarded, and 32 pancreata transplants from DCD donors were performed. These numbers point to the need to optimize DCD protocols, including matching to appropriate recipients. DCD lungs are used efficiently, and only 5.6% were discarded. Pediatric DCD donors also provide quality organs for transplantation.29 Although DCD donors extend the donor pool, they are associated with increased recipient complications, decreased graft survival, and increased cost of transplantation.30 DCD livers, for example, have consistently poorer outcomes than brain-dead donors’ livers, but careful selection of both donors and recipients for these marginal grafts can still help expand the donor pool.31
Living Kidney Donors
nephrectomy donors, probably reflecting attempts to maintain renal perfusion during the period of compromise. Fluid loading overnight before surgery (vs. fluid administration starting with surgery) is associated with better creatinine clearance during the procedure, but this advantage is lost by postoperative day 2,35 likely because donors have good renal function at baseline and the period of renal perfusion compromise is generally short. A reasonable fluid protocol is to administer crystalloid at 10 mL/kg/hr above calculated losses and to maintain urine output at about 100 mL/hr36 or to titrate fluids to a CVP end point, although CVP may not accurately reflect volume status with the patient in lateral decubitus and with pneumoperitoneum.37 Nitrous oxide is contraindicated for laparoscopic donor nephrectomy because distended bowel can get in the way of the surgeons.38 For patient comfort, central venous lines (if used) are generally placed after induction of anesthesia.
Table 51-2. University of Wisconsin Scoring Tool to Predict Breathing After Withdrawal of Care in Potential Donor After Cardiac Death | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Donor nephrectomy should be an uncomplicated procedure and donor tracheas can be extubated in the OR. Deep venous thrombosis prophylaxis is warranted.39 For open nephrectomy, the patient is positioned in the lateral decubitus position with the bed flexed to expose and arch the flank. Donors are generally managed with general anesthesia, but epidural and combined epidural–spinal techniques (supplemented with intravenous propofol)40 as well as general–epidural combined techniques are used. Postoperative pain following donor nephrectomy can be severe, and patient-controlled analgesia is often used. But the pain can still be severe enough to limit respiratory effort and mobilization of the patient. Furthermore, a survey of 123 donors showed that one-third of them had chronic pain after the open procedure,41 suggesting postoperative pain management is often not optimal. Early complications include pulmonary (atelectasis, pneumothorax, pneumonia), urinary tract infections, and wound problems; long-term complications include reduced renal function, hypertension, albuminuria, and psychiatric issues (anxiety, depression).39 Some centers admit donors to a step-down or medical ICU for a day after surgery, but the total hospital stay is usually only 2 to 4 days. Bladder catheters are removed on postoperative day 1. Patients should be advised that full recovery (i.e., feeling normal) takes 4 to 6 weeks, especially after the open procedure. Fortunately, perioperative mortality is rare but cannot be denied as a possible outcome during preoperative patient discussions.
Living Liver Donors
Large liver resections may require virtually complete hepatic venous exclusion (cross-clamping of the hepatic pedicle usually without cava clamping). Not unexpectedly, venous return falls significantly. Without the collaterals developed by patients with chronic liver disease, normal donors may experience significant hypotension when the hepatic pedicle is cross-clamped. Blood pressure is maintained largely through reflex increases in endogenous vasopressin and norepinephrine levels.45 For these reasons, volume loading is reasonable prior to clamping, but some centers try to reduce blood loss by maintaining low CVP, while CVP monitoring is not routine in other centers. Sufficiently powered studies to prove that the benefits of low CVP (reduced transfusion requirements) outweigh the risks (renal compromise, air embolism) are unlikely to be performed, and institutional practices vary widely. If vasopressors are needed, vasopressin and norepinephrine are reasonable choices to enhance normal endogenous reflexes. Isovolemic hemodilution has been reported to reduce allogeneic red cell requirements in major hepatic resections.46 At experienced centers, blood loss is usually <1 L, and 20% to 40% of patients require transfusion.47,48,49 Blood salvage is useful, and some centers offer autologous donation programs for donors. Both can reduce the need for allogeneic blood transfusions.50 Transesophageal echocardiography (TEE), if expertise is available, is ideal and may obviate central line placement. Most donor tracheas can be extubated safely in the OR. Hypothermia is a preventable reason for not extubating in the OR. A wide variety of general anesthetics are used for liver donors, although one small single-center trial suggested that desflurane resulted in better hepatic and renal functions after right liver donation than propofol–remifentanil anesthesia.51
Postoperative pain management in these patients is a matter of controversy because of perioperative coagulopathy (see Chapter 57). Some institutions use epidural catheters, providing excellent analgesia without complications. However, with large liver resection, INR rises significantly after surgery, peaking at 1 day52 or within a few days after surgery, at a time when the catheter is usually removed. (Platelet counts also fall after large liver resections but usually remain in normal ranges.) For this reason, many centers will not place epidural catheters in right liver donors and rely on IV patient-controlled analgesia to manage postoperative pain. Laparoscopic resection of left lobe liver (segments II and III)53 and right lobe54 is available in some centers.
Hypophosphatemia (with excessive loss of phosphate in the urine) is common after hepatectomy,55 and should be treated with sodium phosphate infusions to maintain phosphate levels 3.5 to 5.4 mg/dL, unless patients have significant renal compromise (creatinine clearance <50 mL/hr). Liver function tests other than INR are also abnormal in the postoperative period after liver resection and usually return to baseline levels within 3 months, although small changes in liver function tests can persist for up to a year after hepatectomy. Some living liver donors can experience chronic low platelet counts after hepatectomy.56
Living Lung Donors
Living lung donation is not a common procedure. Selection criteria for living lung donors are listed in Table 51-3.57 Reported morbidity is low,58 but follow-up of these donors is not well reported. If two donors are used for one recipient, scheduling difficulties are considerable. The surgical procedure is performed in three ORs simultaneously, one for each donor and the recipient. Donors are generally managed with combined general anesthesia and epidural analgesia.
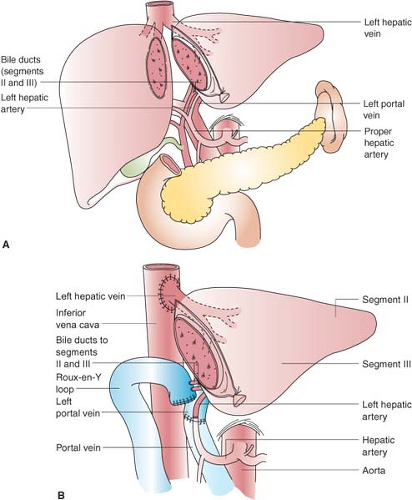 Figure 51-1. Left lateral segment (segments II and III) living donor transplantation. A: Donor operation. B: Recipient operation complete. |
Table 51-3. Living Lung Donor Criteria | ||
|---|---|---|
|
Immunosuppressive Agents
the transplant team needs information about peak and trough drug levels that may not be accessible on the hospital record.
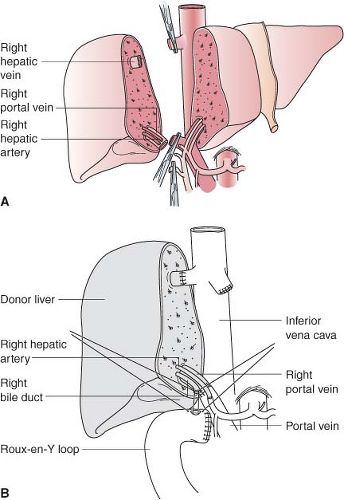 Figure 51-2. Right lobe (segments V to VIII) living donor transplantation. A: Donor operation. B: Recipient operation completed. |
Immunosuppressed patients coming to the OR deserve special attention to sterile technique and maintenance of antibiotic, antifungal, and antiviral regimens during the perioperative period. Complications of chronic immunosuppression are summarized in Table 51-4.
Calcineurin Inhibitors
The modern transplant era began with the introduction of the calcineurin inhibitor (CNI) cyclosporine into clinical practice. CNIs are still a mainstay of immunosuppression for solid-organ transplant recipients. Tacrolimus (FK-506) is also widely used. Inhibition of calcineurin, among other effects, modifies NFAT (nuclear factor of activated T cells) and frees nuclear factor-κB to translocate to the nucleus, where it enhances transcription of T-cell interleukin-2 (IL-2). Via these signal transduction pathways, CNI inhibits T-lymphocyte activation, differentiation, and cytokine production.59 Calcineurin is involved in diverse cellular processes, so its inhibition can cause many significant side effects. These include hypertension (often requiring therapy), hyperlipidemia, ischemic vascular disease (including in heart recipients), diabetes, and nephrotoxicity. Cyclosporine causes acute nephropathy, which is usually reversible with drug cessation. But chronic renal damage from cyclosporine is a more refractory problem. A variety of other drugs are used with CNI to reduce the amount of CNI required. Ischemic cardiac disease is the leading cause of death of kidney transplant recipients, in part because of underlying disease that preceded transplantation. But CNI can exacerbate risk factors for coronary artery disease. It is important to note that end-stage liver disease (ESLD) does not confer protection from coronary artery disease, and liver transplant patients are also at risk for progression of ischemic cardiac disease after transplantation. Neurologic side effects also complicate CNI therapy, including tacrolimus-induced polyneuropathy60 and encephalopathy.61 When immunosuppression is interrupted by surgery or when multiple potentially interacting drugs are used, tacrolimus trough levels should be reassessed.
Table 51-4. Complications of Chronic Immune Suppression | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Tacrolimus is metabolized by cytochrome P450 3A4 and causes its up-regulation. Cyclosporine may rarely prolong the action of pancuronium.62,63 To switch from oral to intravenous dosing of cyclosporine, usually about one-third the oral dose is used. Usual doses of tacrolimus are 0.15 to 0.3 mg/kg/day given in two doses. To switch from oral to intravenous tacrolimus, a starting dose of about one-tenth the oral dose can be used.
Corticosteroids
Corticosteroids disrupt expression of many cytokines in T cells, antigen-presenting cells, and macrophages. These drugs are used both for maintenance immunosuppression and in pulse dosing for acute rejection. Especially for growing children, corticosteroid-sparing regimens are increasingly popular. The well-known side effects are hypertension, diabetes, hyperlipidemia, weight gain (including Cushingoid features), and gastrointestinal ulceration (see Chapter 49). Communication with the transplant service is important in determining timing and dose of steroid administration perioperatively in patients who are taking the drug chronically. Corticosteroids are generally withheld during liver transplantation in recipients with hepatitis C because of concern that they contribute to hepatitis C recurrence.64 Nonetheless, a recent multicenter study showed little advantage to a steroid-free immunosuppression regimen in patients with hepatitis C followed for 2 years after their transplants.65
Polyclonal and Monoclonal Antibodies
Antithymocyte globulin (ATG) is purified rabbit immunoglobulin-G (IgG) taken from animals immunized with human thymocytes, so that previous exposure to rabbits is a risk factor for serum sickness with ATG exposure. Polyclonal ATG suppresses the immune system by depleting immune cells, mostly T cells, but it interacts with a wide variety of cell surface molecules involved in adhesion and cell trafficking and ischemia–reperfusion injury. It has a long history of use in treating acute rejection and induction of immunosuppression, particularly in sensitized transplant recipients and in steroid- and calcineurin-sparing regimens.66 Anesthesiologists should be aware that acute and severe serum sickness is a rare side effect of ATG administration,67 presenting as jaw pain, and is treated by stopping the drug, plasmapheresis, and corticosteroids.68
OKT3 antibody is directed against a component of the T-cell receptor complex and affects immunosuppression by blocking T-cell function. Acute administration of OKT3 in awake patients (especially first administration) may result in generalized weakness, fever, chills, and some hypotension. More severe hypotension, bronchospasm, and pulmonary edema have been reported. Formulations of OKT3 may require syringe filtering before administration.
“Humanized” antibodies are antibodies engineered to contain human constant regions in the immunoglobulin protein, so that patients do not develop an antimouse immunologic response. Muromonab-CD3 is a humanized form of OKT3, usually used for acute rejection.69
IL-2 receptor (CD25) antagonists, such as basiliximab and daclizumab, are newer humanized antibodies directed against a portion of the IL-2 receptor expressed on activated T cells. About half of the heart transplant centers now use IL-2 receptor antagonists in their initial immunosuppression regimen to reduce the dose and cardiovascular side effects of calcineurin inhibitors. Gastrointestinal upset is the most commonly cited side effect of these drugs. However, basiliximab has been implicated in causing pulmonary edema in young renal transplant patients.70
Belatacept is directed against the CD80/CD86 ligands on antigen-presenting cells that activate T cells through the CD28 (costimulatory) pathway. It is approved for maintenance immunosuppression for kidney transplant recipients. Infusion reactions can include hypotension, but acute reactions are usually mild.71 New targets for immunosuppressive drugs include complement and B cells as well as drugs directed at molecules that exacerbate ischemia–reperfusion.72 Coagulopathy has been reported as a side effect of alemtuzumab (Campath),73 which recognizes CD52 on B cells. Rituximab is another anti–B-cell antibody that acts at CD20 and is used for humoral rejection.
Tolerance of organ grafts without pharmacologic immunosuppression is possible using combined bone marrow and solid-organ transplants, with marrow and the solid organ derived from the same donor.74
Other Immunosuppressive Drugs
Mammalian target of rapamycin (mTOR) inhibitors are often used in combination with CNI to decrease the complications of dose-related side effects (calcineurin-sparing regimens) such as nephrotoxicity. TOR is involved in complex signaling processes that promote synthesis of proteins, including several that regulate cellular proliferation. Thus, mTOR inhibitors such as sirolimus (Rapamycin) are antiproliferative, used both in immunosuppression and increasingly in cancer therapies. Similar to cyclosporine and tacrolimus, sirolimus is metabolized in liver via P450 CYP3A isoenzymes, but coadministration of sirolimus and a calcineurin inhibitor does not increase calcineurin inhibitor drug requirements. In fact, the combination may be synergistic.75 Diltiazem raises the plasma concentration of sirolimus.76
Azathioprine is hydrolyzed in blood to 6-mercaptopurine, a purine analog and metabolite with the ability to incorporate into DNA during the S phase of the cell cycle. Because DNA synthesis is a necessary prerequisite to mitosis, azathioprine exerts an antiproliferative effect. Antiproliferative drugs rely on the fact that immune activation implies explosive proliferation of lymphocytes. Side effects occur because other proliferating cells (gastrointestinal tract, bone marrow) are also affected. Repression of bone marrow cell cycling can cause pancytopenia. Cardiac arrest and severe upper airway edema are rare complications.77 The intravenous dose is about half the oral dose.
Mycophenolate mofetil is metabolized into a molecule that inhibits purine synthesis. It too can cause leukopenia and thrombocytopenia as side effects, as well as red cell aplasia78; additionally it is teratogenic.79 The usual oral dose is 1 to 1.5 g twice a day. Mycophenolate has largely replaced azathioprine in combination regimens.80
Mesenchymal stem cells (MSCs) are anti-inflammatory stem cells, currently in multiple clinical trials for solid-organ transplantation. MSCs are multipotent cells that are easily derived from marrow, fat, or other organs and expanded ex vivo. Their first use in the context of organ transplantation was for the treatment of grade IV graft versus host disease in marrow transplant recipients.81 MSCs have pleiotropic effects on the immune response, including antiproliferative T-cell function. The goal of MSC trials for solid-organ transplantation is to reduce the amount of pharmacologic immunosuppression needed. Although these are allogeneic cells, they do not elicit a classical immune response, so third-party MSCs can be used in any patient without the need for donor-specific MSC. No adverse acute reactions to MSC infusion or injection have been reported after thousands of treatments.
Corneal Transplantation
Corneas are the most common organs transplanted in the United States, with >40,000 transplants per year. Corneal transplants can be performed under local anesthesia (often with IV sedation) or general anesthesia. Recipients of cornea grafts are often elderly. A major anesthetic goal is maintaining low intraocular pressure (see Chapter 48 – EYE). For particularly complex procedures, diuretics and intravenous fluid restriction may be warranted to prevent edema.82 Patient positioning (head-up angle of the bed) on transfer from the OR should be directed by the surgeon. Rejection of corneal allografts is the most common cause of graft loss in the first year after transplantation.
Renal Transplantation
Preoperative Considerations
The prevalence of end-stage renal disease (ESRD) is about 0.17% in the United States, where 16,899 kidney transplants were performed in 2010, representing no change in transplant volume in 5 years despite growing need. The most common recipient age group was 50 to 64 years (6,684 patients), and 2,813 recipients
were over 65 years. Thirty-seven percent overall received living donor grafts. Mortality on the waiting list in 2009 was 6.3%.c
were over 65 years. Thirty-seven percent overall received living donor grafts. Mortality on the waiting list in 2009 was 6.3%.c
Table 51-5. Diagnoses of Patients on Adult Renal Transplant Waiting List | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Diseases treated with renal transplants are shown in Table 51-5, calculated from the 2008 waiting list data. Many of these underlying diagnoses are also risk factors for coronary artery disease, so preoperative evaluation is focused on cardiovascular function, but a comprehensive medical workup is essential.
All solid-organ transplant patients are screened for tumors (mammography, Papanicolaou [Pap] test, colonoscopy, prostate-specific antigen) and infection (dental evaluation, viral serologies). Patients should have good control of their diabetes before transplantation and have an evaluation for psychiatric stability and social support. Severe heart, lung, or liver disease, most malignancies, and active or untreatable infections such as tuberculosis are exclusion criteria for renal transplantation.
Dialysis-dependent patients should be dialyzed before surgery. Cadaveric grafts can be safely transplanted after 24 hours of cold ischemia time, and potentially after 36 hours, allowing scheduling of preoperative dialysis. With preoperative dialysis, severe hyperkalemia during surgery is unusual.
ECDs are often used for kidney transplantation. Factors used in designation of ECD kidneys are age, creatinine, stroke as cause of death, and hypertension. Healthy older living donor kidneys have outcomes similar to standard criteria donor kidneys.86 For example, donors over 50 years old are considered ECD if they have a history of hypertension, creatinine >1.5 mg/dL, or cause of death was cerebrovascular accident.87 DCD donors are also an ECD category. ECDs affect the scheduling of transplantation as minimization of cold ischemia times is essential and delayed graft function may complicate the intraoperative course of DCD graft recipients.88 Some centers report increased rates of primary nonfunction in DCD kidney grafts, contributing to early graft loss.89
Kidney allocation is a bit more complex than liver allocation. Blood type O kidneys are allocated to type O recipients, and blood type B kidneys are transplanted only in B recipients, except in the case of zero antigen mismatched candidates. Zero mismatched kidneys have the same HLA A, B, and DR antigens, and there is mandatory sharing of these kidneys through UNet with a complicated set of rules governing priority for these donors (see http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_7.pdf).
Intraoperative Protocols
Renal transplantation is generally done under general anesthesia. Small studies have suggested good outcomes with epidural anesthesia90 or combined epidural–spinal anesthesia. But concerns over uremic platelet dysfunction and residual heparin from preoperative dialysis have limited the use of regional anesthesia for kidney transplantation. Rapid-sequence induction is indicated in diabetic patients with gastroparesis (preceded by oral sodium bicitrate). Anemic, hyperdynamic patients may have higher dose requirements for induction agents such as propofol.91 Rocuronium is useful for patients in whom rapid-sequence induction is indicated, but the duration of block is variable in patients with ESRD.92 Similarly, plasma clearance of rapacuronium is reduced with renal failure, but titration of dose to neuromuscular blockade monitoring end points prevents delayed recovery.93 Generally, the long-acting nondepolarizing muscle relaxants are avoided and shorter-acting agents such as cisatracurium are used. Before incision, antibiotics are given. A central venous catheter (usually triple lumen) is placed for CVP monitoring and drug administration in most centers, and a bladder catheter is placed.
Incision is usually in the lower right abdomen to facilitate placement of the graft in the iliac fossa. The recipient iliac artery and vein are used for graft vascularization, followed by connection of the ureter to the recipient bladder. If the kidney is too large for the iliac fossa, it can be positioned in the retroperitoneal space. Iliac vessels may be used for anastomoses, or the aorta and inferior vena cava may be required.
Hypertensive renal transplant patients often require antihypertensive drugs perioperatively. Calcium channel blockers have
been best studied for renal protection of cyclosporine-treated hypertensive transplant patients. But after surgery, angiotensin-converting enzyme inhibitors and α-blockers may be as effective as calcium channel blockers.95 Typical hemodynamic goals during transplant are systolic pressure >90 mm Hg, mean systemic pressure >60 mm Hg, and CVP >10 mm Hg. These goals are usually achievable without vasopressors, using isotonic fluids and adjustment of anesthetic doses. Hemodynamic management varies widely from center to center, so close communication between surgeon and anesthesiologist is imperative. Plasma-Lyte is the crystalloid of choice for kidney transplantation, and it preserves acid–base balance and electrolytes when compared with Ringer’s lactate or normal saline.96
been best studied for renal protection of cyclosporine-treated hypertensive transplant patients. But after surgery, angiotensin-converting enzyme inhibitors and α-blockers may be as effective as calcium channel blockers.95 Typical hemodynamic goals during transplant are systolic pressure >90 mm Hg, mean systemic pressure >60 mm Hg, and CVP >10 mm Hg. These goals are usually achievable without vasopressors, using isotonic fluids and adjustment of anesthetic doses. Hemodynamic management varies widely from center to center, so close communication between surgeon and anesthesiologist is imperative. Plasma-Lyte is the crystalloid of choice for kidney transplantation, and it preserves acid–base balance and electrolytes when compared with Ringer’s lactate or normal saline.96
Once the first anastomosis is started, diuresis is initiated (both mannitol and furosemide are often given). Heparin and verapamil should also be available in the OR. In some centers, anesthesiologists are asked to administer the first doses of immunosuppression. A kidney graft is defective in concentrating urine and reabsorbing sodium, so attention to electrolytes is important.
Glucose control is also important for patients undergoing transplantation. A small, single-center prospective study of living kidney donor recipients identified glucose >160 mg/dL as a risk factor for acute perioperative renal dysfunction, likely associated with more severe ischemia–reperfusion injury.97 Tight glucose control after kidney transplant is associated with less rejection, and diabetics with poorly controlled glucose levels after transplantation have increased mortality.98 For these reasons, tight blood glucose control (80 to 110 mg/dL) is a reasonable anesthetic management goal during renal transplantation. Dopamine does not reliably improve renal function in this setting. The selective DA1 agonist fenoldopam is used to preserve renal function during kidney transplantation in some centers and is a superior renal protectant,99 although not extensively studied.
Transfusion is rarely required in the OR, although renal transplant patients are often anemic coming to surgery (and may be receiving erythropoietin). Because of immunosuppression, if cytomegalovirus (CMV)-negative patients receiving a CMV-negative organ are transfused, CMV-negative blood is preferred. Leukocyte filters are also effective in preventing CMV transmission but are probably inferior to CMV-negative blood.100 The entire surgery should take about 3 hours.
Most surgical complications of renal transplantation are not recognized in the OR. The common postoperative complications are ureteral obstruction and fistulae, vascular thromboses, lymphoceles, wound complications,101 and bleeding.
Patient-controlled analgesia is a good choice for postoperative pain management, and despite prolonged action, morphine can be used safely if patients are monitored intensively. Nonsteroidal anti-inflammatory agents and cyclooxygenase-2 inhibitors are contraindicated. Pain can be severe, prompting some centers to explore combination blocks (ilioinguinal–iliohypogastric and intercostal nerve blocks)102 or transversus abdominis plane blocks103 for posttransplant patients. Chronic pain after kidney transplantation is common,104 suggesting that more attention should be given to early postoperative pain management. Kidney transplant recipients are generally discharged from the hospital within a week of surgery.
In children, the most common causes of ESRD requiring transplantation are congenital (largely anatomic developmental) anomalies. Only 15% of pediatric transplants are performed for children <2 years of age.105 Kidney size mismatch can complicate the surgery in small children. Adult donor kidneys may have to be placed in the retroperitoneum of small children. Although chronic peritoneal dialysis may help expand the abdominal volume,106 attention to peak inspiratory pressures at closure is important, and increased pressures should be reported to the surgical team. Pediatric renal transplantation is associated with somewhat lower rates of success than adult transplantation, with vascular thromboses of the grafts more common in younger children.
Liver Transplantation
Preoperative Considerations
Adult liver transplant recipients are prioritized for transplantation by severity of illness, using the MELD (model for ESLD) score, which was originally developed to predict survival of patients with liver disease independent of liver transplantation107:
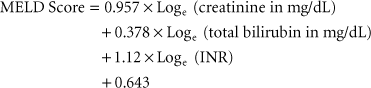
Pediatric patients are prioritized for transplant using the pediatric ESLD score (PELD):
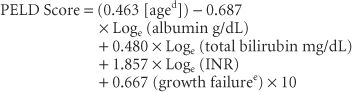
Patients with acute liver failure are given priority for donor livers, then the patients with the highest MELD score and compatible blood group are next.
All liver transplant patients are screened for infectious diseases including human immunodeficiency virus (HIV), CMV, and Epstein-Barr virus. As for other solid-organ transplants, major infection and malignancy may exclude patients from consideration for transplantation. Several centers have had good experience transplanting patients who have HIV, and HIV-infected patients who require liver transplants should be referred to these centers.108
Renal dysfunction is common in patients undergoing liver transplantation. Serum creatinine levels are not extremely useful in capturing renal function in patients with liver disease. Even a small increase in serum creatinine in these patients suggests significant renal dysfunction; hence, the reason that creatinine is emphasized in MELD scoring. Ongoing evaluation of MELD/PELD scores of
wait-listed patients is mandated, with the sickest patients requiring the most frequent laboratory studies to update scores.
wait-listed patients is mandated, with the sickest patients requiring the most frequent laboratory studies to update scores.
Table 51-6. Multisystem Complications of End-stage Liver Disease | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Difficult decisions about patient candidacy are common in evaluating liver transplant candidates. Several are discussed here to highlight the need for regular involvement of a transplant anesthesiologist in the candidacy evaluation process. Patients with ESLD generally have very low SVR, high cardiac index, and increased mixed venous oxygen saturation. Liver disease is not protective against coronary artery disease. Most patients are screened for cardiac disease using dobutamine stress echocardiography or myocardial stress scintigraphy, although the effectiveness of diagnosing coronary artery disease in these patients is not well established.109 Patients with evidence of significant coronary lesions usually require cardiac catheterization to identify stenoses amenable to angioplasty preoperatively. These studies can be done safely even in patients with significant renal dysfunction.110 Patients with severe coronary artery disease are generally not candidates for liver transplantation. Functional assessment of patients in addition to laboratory and imaging studies is important, and one study found that a distance of <250 m on a 6-minute walk test is an independent predictor of death on the transplant waiting list.111 A recent, small retrospective studies suggests that troponin I levels >0.07 ng/mL may predict liver transplant patients at risk for cardiovascular complications.112
Significant aortic stenosis also presents a difficult dilemma pretransplant. Because cardiac surgery is considered risky in a patient with ESLD, patients with aortic stenosis can be treated with valvuloplasty before liver transplantation. Then, after the liver graft is stable, aortic valve replacement can be considered. Similarly, patients with hypertrophic obstructive cardiomyopathy (HOCM) and left ventricular outflow tract obstruction can be treated with alcohol ablation of the septum before surgery to improve cardiac function during transplantation.113 These HOCM patients can benefit particularly from TEE monitoring because PA capillary wedge pressure does not accurately reflect left ventricular (LV) volume in this population. Patients with patent foramen ovales may be at risk for intraoperative stroke, and some centers work with cardiologists to close larger shunts noninvasively before surgery.
Table 51-7. Diagnoses Leading to Liver Transplantation in Adults | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Portopulmonary hypertension (PPH) patients coming to transplant are particularly challenging. Diagnosis of PPH is made in the (a) setting of liver disease (b) mean positive airway pressure ≥25 mm Hg, pulmonary vascular resistance (PVR) >240 dyne/s/cm5, and PA occlusion pressure ≤12 mm Hg.114 Echocardiography is also used to screen patients for portopulmonary hypertension and intracardiac shunts. Systolic PA pressure estimates are made by capturing the maximum velocity of regurgitant flow across the tricuspid valve, and this velocity is used in the Bernoulli equation for the pressure gradient between the right ventricle and the right atrium (ΔP = 4V4). If moderate to severe pulmonary hypertension (estimated systolic PA pressure >50 mm Hg) is suggested, right heart catheterization is needed for direct pressure measurements. Multiple case reports and small retrospective reviews demonstrate that patients with portopulmonary hypertension are at substantial risk of perioperative death. There is general agreement that a mean PA pressure >50 mm Hg is an absolute contraindication to liver transplantation. Patients with PA pressures between 35 and 50 mm Hg and pulmonary vascular resistance >250 dyne/s/cm−5 are also likely at increased risk. Efforts to lower PA pressure before transplantation pay off and considerably reduce the risk of transplantation.115 Epoprostenol is the usual first-line therapy for portopulmonary hypertension and is effective in lowering PA pressures significantly in many patients, but it requires home IV delivery. Inhaled iloprost has been used in Europe for PPH patients with good results.116 Sildenafil is also useful for treatment of portopulmonary hypertension117 and can be given via nasogastric tube during surgery. Controlling PA pressures is critical in patients with the most severe PPH, and a few patients have been successfully managed with the mixed endothelin antagonist bosentan118 or the selective endothelin-A receptor antagonist ambrisentan (Cartin-Ceba) or imatinib.119 Right heart dysfunction that does not reverse after treatment of primary pulmonary hypertension is considered a contraindication to liver transplantation.120 Inhaled nitric oxide is extremely useful for managing PA pressures during liver transplantation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





