CHAPTER 91 TRANSFUSION: MANAGEMENT OF BLOOD AND BLOOD PRODUCTS IN TRAUMA
Approximately 15% of all blood transfusions in the United States are used in the care of patients that have sustained traumatic injury. Blood transfusion in trauma is life-saving for those patients in hemorrhagic shock who are unresponsive to crystalloid fluid resuscitation. Importantly, concomitant attempts at prompt cessation of hemorrhage are also necessary. Blood transfusion in trauma has also been identified as an independent predictor of multiple-organ failure (MOF), systemic inflammatory response syndrome (SIRS), increased postinjury infection, and increased mortality in multiple studies. The cumulative risks of blood transfusion have been related to the number of units of packed red blood cells (PRBCs) transfused, increased storage time of transfused blood, and possibly donor leukocytes. Lack of efficacy of red blood cell transfusion in critically ill patients has also been documented.1 Therefore, once hemorrhage control has been established in acute trauma we should attempt to minimize the use of blood transfusion for the treatment of asymptomatic anemia in trauma patients.
A number of potential mechanisms that may mediate adverse affects associated with blood transfusion in trauma have been proposed, including increased systemic inflammatory response, immunomodulation, microcirculatory dysfunction due to altered RBC deformability, increased nitric oxide binding by free hemoglobin and vasoconstriction, and others. These data have led some to conclude that blood transfusion in the injured patient should be minimized whenever possible.2
INCIDENCE: WHO NEEDS BLOOD TRANSFUSION IN TRAUMA?
Trauma patients in hemorrhagic shock have an absolute indication for PRBC transfusion if they are unresponsive to isotonic crystalloid fluid resuscitation, have ongoing significant hemorrhage, and manifest physiologic signs of persistent shock (hypotension, tachycardia, oliguria, lactic acidosis, abnormal base deficit)—indicating that oxygen consumption is dependent on hemoglobin concentration (critical oxygen delivery).3 In these patients, the prompt transfusion of PRBCs in conjunction with prompt hemorrhage control can be life-saving.
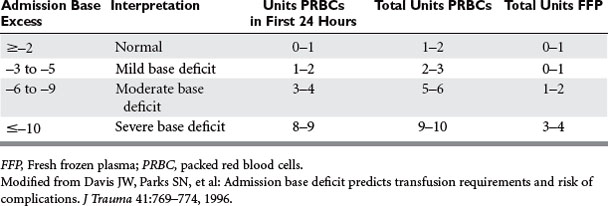
Patients with hemorrhagic shock, identified by a metabolic acidosis and increasing base deficit, have been documented to require increased blood and plasma transfusion (Table 1). A single-institution study in calendar year 2000 documented that 8% (479 of 5645) of acute trauma patients received PRBCs, using 5219 units. The majority (62%) of transfusions were administered in the first 24 hours of care. Only 3% of patients (n = 147) received more than 10 units of PRBCs, and these patients also received plasma and platelet transfusions to treat actual or anticipated dilutional coagulopathy. Mortality rates in trauma patients who require blood transfusion is high, ranging from 27% to 39%.4
A recent study identified independent risk factors for blood transfusion in trauma (n = 1103), including increased age, admission from scene, trauma mechanisms of motor vehicle crash or fall from height, admission systolic blood pressure <120 mm Hg, free fluid on abdominal ultrasound, and unstable pelvis on clinical examination (Table 2). The probability of RBC transfusion in the emergency department was significantly increased if these risk factors were present, and increased substantially with multiple risk factors. An Emergency Room Transfusion Score (ETS) was calculated from these rapidly assessable parameters, ranging from 1 point to 9.5 points maximum. The probability of blood transfusion exponentially increased with the sum of points in the ETS (i.e., from 0.7% at 1 point to 5% at 3 points and 97% at the maximum of 9.5 points).5
Table 2 Risk Factors Associated with Blood Transfusion in Emergency Room After Severe Trauma
| Variable | Score | Odds Ratio (95% CI) |
|---|---|---|
| Age (Years) | ||
| 0–20 | 0 | 2.0 (0.9–4.3) |
| 20–60 | 0.5 | 5.6 (2.3–13.4) |
| >60 | 1.5 | |
| Admission | ||
| From scene | 1.0 | 2.4 (1.3–4.5) |
| From other hospital | 0 | |
| Trauma Mechanism | ||
| Traffic | 1 | 3.2 (1.7–6.0) |
| Fall from height >3 m | 1 | 2.4 (1.1–5.2) |
| Blood Pressure (mm Hg) | ||
| 0–90 | 2.5 | 12.2 (6.4–23.4) |
| 90–120 | 1.5 | 4.1 (2.3–13.4) |
| >120 | 0 | |
| Abdominal Ultrasound | ||
| Free fluid | 2 | 8.4 (4.3–16.2) |
| No fluid | 0 | |
| Pelvis on Clinical Examination | ||
| Unstable | 1.5 | |
| Stable | 0 | 4.7 (2.1–10.3) |
| Total risk factor points | 9.5 | |
Adapted from Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR: Blood transfusion rates in the care of acute trauma. Transfusion 44(6):809–813, 2004.
For severe hemorrhagic shock, type O blood should be transfused.6 Rh-negative blood should be used in women of childbearing age if possible. A prompt transition to the use of type-specific (ABO, Rh-matched) blood should be accomplished as quickly as possible, and use is continued until fully cross-matched units of blood are available. Once a trauma patient has been administered more than one blood volume and the initial antibody screen is negative, there is no point attempting compatibility testing except for ABO matching.
Once hemorrhage control has been established and the patient has completed resuscitation from hemorrhagic shock, all efforts to restrict RBC transfusion in trauma are advisable. Anemia is common in critically injured trauma patients and persists throughout the duration of critical illness, as documented in a post hoc analysis of a subset of trauma patients (n = 576) from a prospective multicenter observational cohort study in the United States.7 While in the intensive care unit (ICU), 319 (55.4%) trauma patients received a total of 1858 units of blood (or 5.8 ± 5.5 units of blood each) on average. The majority (87.1%, n = 278) of all ICU trauma patients requiring transfusions received them within the first 4 days, accounting for almost half of all ICU transfusions. However, 5%–10% of patients continued to receive blood transfusions. Importantly, this study documented that a large number of blood transfusions were administered when the hemoglobin concentration was greater than 10 g/dl.
National guidelines regarding blood transfusion differ, such that the American Society of Anesthesiology8 recommends maintaining hemoglobin (Hb) greater than 6 gm/dl—whereas the National Institutes of Health9 (NIH) recommends maintaining Hb greater than 7 g/dl for patients who are critically ill. A Cochrane Database Systematic Review titled Transfusion Thresholds and Other Strategies for Guiding Allogeneic Red Blood Cell Transfusion concluded that the limited published evidence (10 trials, n = 1780 patients) supports the use of restrictive transfusion triggers (blood transfusion only if Hb <7 g/dl) in patients who are free of cardiac disease.10
An analysis from the prospective multicenter randomized controlled trial (Transfusion Requirements in Critical Care, TRICC) compared the use of restrictive (transfuse if Hb <7 g/dl) and liberal (transfuse if Hb <10 g/dl) transfusion strategies in resuscitated critically ill trauma patients (n = 203). The average hemoglobin concentrations (8.3 ± 0.62 g/dl vs. 10.4 ± 1.2 g/dl; p < 0.0001) and the RBC units transfused per patient (2.3 ± 4.4 vs. 5.4 ± 4.3; p < 0.0001) were significantly lower in the restrictive group than in the liberal group. No differences in mortality, multiple-organ dysfunction, or ICU or hospital length of stay were identified—suggesting that a restrictive RBC transfusion strategy appears to be safe for critically ill multiple trauma patients.11
RISKS OF BLOOD TRANSFUSION
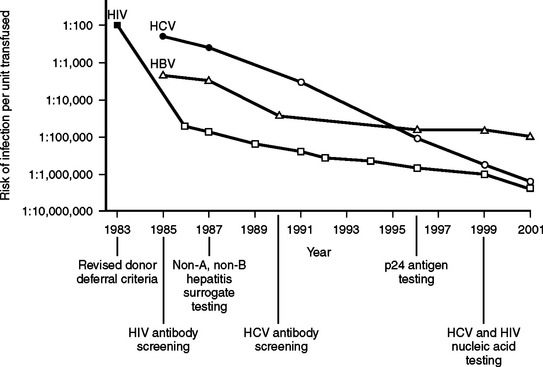
The blood supply in the United States has never been as safe as it is now. During the past several decades, there have been dramatic progressive reductions in the risk of transfusion-transmitted clinically significant blood-borne infections.12 Figure 1 summarizes the significant decline in human immunodeficiency virus (HIV), hepatitis B (HBV), and hepatitis C (HCV) risks of transmission through blood transfusion.
Although there has been a 10,000-fold reduction in the risk to patients from transfusion-transmitted infectious diseases in recent decades, there has been little progress in reducing the risk of noninfectious hazards of transfusion. A recent analysis of 366 spontaneously reported deaths and major complications of transfusion in the United Kingdom and Ireland identified that the majority (53%) of these adverse events were related to the incorrect blood component being transfused (i.e., a clerical or human-related error).13 As a result, patients today are harmed from noninfectious serious hazards of transfusion at a rate that exceeds infectious hazards by 100-fold to 1000-fold (Table 3).
Table 3 Risks Associated with Transfusion
| Type of Risk | Incidence |
|---|---|
| Noninfectious Risks | |
| Depressed erythropoiesis | Universal |
| Volume overload, pulmonary edema | 10%–40% |
| Febrile reaction | 1/10–1/100 |
| Urticarial reaction | 1/33–1/100 |
| Transfusion-related acute lung injury (TRALI) | 1/1120–1/5000 |
| Delayed hemolytic transfusion reaction | 1/2500 |
| Hemolytic transfusion reactions | 1/38,000–1/70,000 |
| Fatal hemolytic transfusion reactions | 1/600,000 |
| Anaphylactic shock | 1/500,000 |
| Immunosuppression | Unknown |
| Graft-versus-host disease | 1/400–1/10,000 |
| Alloimmunization (RBCs) | 1/100 |
| Alloimmunization (platelets) | 1/10 |
| ABO-Rh mismatch | |
| Occurrence | 1/6,000–1/20,000 |
| Mortality | 1/100,000–1/600,000 |
| Infectious Risks | |
| Cytomegalovirus conversion | 7% |
| Epstein-Barr virus | 0.5% |
| Bacterial contamination (PRBCs + platelets) | 1/2000 |
| Hepatitis B transmission | 1/220,000 |
| PRBC-related bacterial sepsis | 1/500,000–1/786,000 |
| Hepatitis A transmission | 1/1,000,000 |
| West Nile Virus transmission | 1/1,400,000 |
| Hepatitis C transmission | 1/1,600,000 |
| HIV transmission | 1/1,800,000 |
Adapted from Klein HG: Allogenic transfusion risks in the surgical patient. Am J Surg 170;6A:21S–26S, 1995; Silliman CC, Moore EE, Johnson JL, et al: Transfusion in the injured patient: proceed with caution. Shock 21(4):291–299, 2004; Busch MP, Kleinman SH, Nemo GJ: Current and emerging infectious risks of blood transfusions. JAMA 289(8):959–962, 2003; and Spiess BD: Risk of transfusion: outcome focus. Transfusion 44:4S–14S, 2004.
Emerging risks of blood transfusion include West Nile virus (WNV) transmission via blood transfusion. Between August of 2002 and January of 2003, there were 23 confirmed cases of transfusionassociated WNV from 14 donors. Experimental WNV nucleic acid amplification test assays were implemented for blood donor testing in July of 2003. Reports through September 2003 indicated that more than 600 infected units of blood were identified from 2.5 million donations during a period of 4137 known WNV infections reported to the Centers for Disease Control and Prevention (CDC).14 Although nucleic acid amplification testing of blood donations prevented hundreds of cases of WNV infection, it failed to detect units with a low level of viremia—some of which were antibody negative and infectious. These data support the use of targeted nucleic acid amplification testing of individual donations in high-prevalence WNV regions, a strategy that was implemented successfully in 2004.15
Transfusion-Related Acute Lung Injury
Transfusion-related acute lung injury (TRALI) is a life-threatening complication of blood transfusion. TRALI is now the leading cause of transfusion-related mortality, even though it is probably still underdiagnosed and underreported. The National Heart, Lung and Blood Institute of the NIH convened a working group on TRALI, and a common definition has been established—defined as new acute lung injury occurring during or within 6 hours after a blood transfusion.16 TRALI, like the acute respiratory distress syndrome (ARDS), may be a two-event phenomenon—with both recipient predisposition and factors in the stored blood units playing major roles.17 The overall prevalence has been reported as 1 in 1120 cellular components transfused.
A recent prospective cohort study documented a significant independent association between the amount of transfused blood and the development of ARDS and hospital mortality. The association between the amount of transfused blood and the development of ARDS remained significant in a multivariable logistic regression model accounting for differences in severity of illness, type of trauma, race/ethnicity, gender, and base deficit.18 A larger study in 5260 blunt trauma patients documented that delayed blood transfusion (defined as no blood transfusion received within the initial 48 hours after admission) was independently associated with ventilator-associated pneumonia, ARDS, and death in trauma regardless of injury severity.19 Similarly, an additional study that examined clinical predictors of ARDS identified that PRBC transfusion was an independent risk factor for increased development of and increased mortality in ARDS.20 All of these studies mandate a judicious transfusion policy after trauma resuscitation, and emphasize the need for safe and effective blood substitutes and transfusion alternatives.
MASSIVE TRANSFUSION
Massive blood transfusion is most commonly defined as complete replacement of a patient’s blood volume within a 24-hour period or more than 10 units of PRBCs in 24 hours. Newer definitions include an ongoing blood loss of more than 150 ml/min, or the replacement of 50% of the circulating blood volume in 3 hours or less.21 These newer definitions have the benefit of allowing early recognition of major blood loss and of the need for effective intervention to prevent hemorrhagic shock and other complications of massive hemorrhage and transfusion.
Massive transfusion therapy for the treatment of hemorrhagic shock requires a coordinated and detailed approach with the fundamental components listed in Table 4. In the absence of a predefined massive transfusion protocol, access to the appropriate blood products (and adequate volume of these products) may be significantly delayed. Without prompt replacement of these blood products, the resultant coagulopathy may worsen and bleeding will continue. In fact, the implementation of an organized “Massive Transfusion Policy” to address exsanguinating hemorrhage in the trauma population has proven of benefit in patient outcomes and in reducing blood product utilization. Studies have demonstrated an increase in survival (16%–45%) in patients with exsanguinating hemorrhage following the implementation of such a protocol.22,23 Guidelines for the treatment of acute massive blood loss are listed in Table 5.
Table 4 Management of Massive Transfusion for Hemorrhagic Shock
Table 5 Acute Massive Blood Loss: Template for Guidelines
| Goal | Procedure | Comments |
|---|---|---|
< div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|