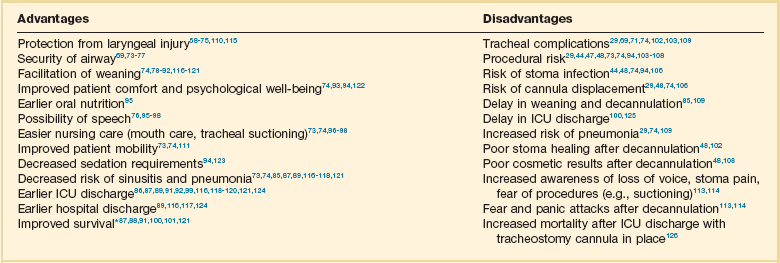
14 Most medications, devices, and surgical techniques employed in today’s intensive care unit (ICU) have resulted from twentieth century medical advances. However, one of the most common surgical procedures, tracheostomy, has been described for nearly 6000 years. The terms tracheostomy and tracheotomy are derived from the Greek words tracheia arteria, translated “rough artery,” and refer to the trachea being the vital conduit of air.1 Tracheostomy means permanent opening (stoma, Greek for “mouth”), to be distinguished from the temporary nature of a tracheotomy (tome, “to cut”). Today the terms are used interchangeably for any artificial airway created in the trachea, with tracheostomy used more commonly. McClelland divides the history of the tracheostomy into five periods beginning with “The Period of Legend” (2000 BCE to 1546 CE).2 The first written reference to tracheostomy is in the sacred book of Hindu medicine, the Rig Veda, dated between 2000 BCE and 1000 BCE, and describes “the bountiful one, who without a ligature, can cause the windpipe to reunite when the cervical cartilages are cut across.”3,4 The earliest depictions of a tracheostomy being performed date back to about 3600 BCE and show two Egyptian kings undergoing a tracheostomy.5,6 Homer referred to tracheostomy as a way of relieving choking persons by cutting open the trachea, and Alexander the Great is described to have used the point of his sword to open the trachea of one of his soldiers who was choking while eating.2 The next successful tracheostomy was performed in the second century CE by Antyllus as documented 400 years later by Paul of Aegina, who wrote that the use of tracheostomy was encouraged in cases of upper airway obstruction and provided a technical description of the operation.7 Mention of tracheostomy can be found in the Roman and Arabic literature, although during the Dark Ages of medicine and science the technique of tracheostomy (and virtually all other surgeries) was forgotten for nearly 1000 years.6,8 The second period, “The Period of Fear” (1536 to 1833),2 starts with the Renaissance, when European physicians experimented with tracheostomy for trauma, aspirated foreign bodies, drowning, and Ludwig’s angina. Fabricius of Aquapendente tells us that the timidity at that time was due partly to “lack of knowledge of anatomy, partly to fear of a loss of reputation, should the patient die after the operation.” In his day, tracheostomy was known as the “scandal of surgery.” About the same time, in 1546, the first definite account of a successful tracheostomy was recorded by Antonius Brasavola, who told of “opening the windpipe and saving the life of a patient near death from angina and an abscess which was obstructing the canalis pulmonis.”1,8 Over the next 2 centuries, opposition to tracheostomy diminished because of the interest in anatomy and autopsy, as evidenced by the drawings and writings of Leonardo da Vinci, Julius Casserius, and others.6,9–11 The clinical importance of tracheostomy became evident during sporadic diphtheria epidemics, and multiple publications of successful tracheostomies can be found in many European countries after 1620.8 In 1730, Scottish surgeon George Martine treated upper airway obstruction resulting from diphtheria with tracheostomy. He also recommended the use of an inner cannula for ease of care and recognized that tracheal wounds heal spontaneously without the need for surgical repair.8,12 In America, fear of tracheostomy was still quite prevalent, as evidenced in the well-known controversy surrounding the death of George Washington in 1799, when bloodletting won over relieving an epiglottitis-related upper airway obstruction with a tracheostomy.6,13 Until 1825, during these first several thousand years in the history of tracheostomy, only 28 tracheostomies are verifiable. After Napoleon Bonaparte’s nephew died of diphtheria in 1807, a grand prize was offered for new insights into this disease and its treatment. This heralded the “Period of Drama,” which lasted from 1833 to 1931. Based on subsequent research, especially by Bretonneau and his pupil Trousseau, tracheostomy became a relatively established procedure, particularly for croup and diphtheria. Before Bretonneau, tracheostomy had been known under many different names, including bronchotomy, laryngotomy, pharyngotomy, sectio epiglottis, scisio cannae, and incisio cannae pulmonis. In 1718 Heister had introduced the term tracheotomy and recommended that all other terms be discarded, but it was not until Bretonneau used this term in a paper describing a successful operation in 1825 for diphtheria that it gained widespread acceptance.8,14 Trousseau described a series of 200 French children, most dying of diphtheria, in whom he reduced mortality rate from nearly 100% to 75% with tracheostomy.15 American surgeon Thomas Shastid published an account of performing tracheostomies on children in the 1890s in a small Illinois town during a diphtheria epidemic.16,17 In the mid-1800s, Snow and Trendelenburg advocated tracheostomy for administration of inhaled anesthetics,3,18 but endotracheal intubation, performed by MacEwan in 187819 and O’Dwyer in 1880,20 and popularized by Bartholomay and Dufor in 1907 and Kelly in 1912, soon replaced tracheostomy as the route for delivering general anesthesia.3,21 This put the performance of tracheostomy squarely in the hands of surgeons experienced in upper airway problems, most notably Chevalier Jackson, whose name became virtually synonymous with tracheostomy. In his hands, the rate of mortality attributable to tracheostomy decreased from 25% to less than 5%.22 The fourth period, the “Period of Enthusiasm” (1932 to 1965), was heralded by a revolutionary shift in the indications for the procedure and major advances in the technique. During this period the main sentiment was “if you think of tracheostomy, do it,” without consideration of possible adverse outcomes. Up to then the main indication for tracheostomy was upper airway obstruction, mostly caused by severe croup and diphtheria, which practically disappeared as a result of the development of antibiotics and immunization. In the 1950s, with the poliomyelitis epidemic in Europe and North America, the need for positive-pressure ventilation (PPV) and tracheobronchial suctioning created new indications for tracheostomy.23 At present, these are still the major indications for tracheostomy in the ICU, and they caused the number of tracheostomies performed at Massachusetts General Hospital to increase from fewer than 10 in 1947 to more than 150 in 1959. Before 1958, not a single tracheostomy was performed there for the sole purpose of providing PPV.24 The exponential increase in tracheostomy in the 1950s and 1960s revived older controversies. In 1921, Chevalier Jackson discussed proper technique and complications.25 He condemned the use of high tracheostomy, believing it caused laryngeal stenosis. For 50 years, this was the prevailing wisdom of neck surgery and airway maintenance. Jackson drew his conclusions, however, primarily from unsterile emergency tracheostomies performed by inexpert practitioners.25 Laryngeal stenosis probably resulted from factors other than the site of incision, especially when performed with preexistent laryngeal disease.26,27 Jackson’s reputation made surgeons reluctant to perform high tracheostomies until the advent of cardiac surgery made them necessary to avoid contaminating median sternotomy incisions. In 1976, two cardiac surgeons, Brantigan and Grow, published the first large series of high tracheostomies and found few complications.28 Acceptance of high tracheostomy permitted reintroduction of PT; a technique described 20 years earlier. As previously described, when ICUs were developed, tracheostomy became one of the most frequently performed procedures in critically ill patients.29 This created a need for a safe, cost-effective bedside procedure that would eliminate the necessity for transport of the patient from the ICU to the operating room, with its attendant risks, which are discussed subsequently. In 1955, Shelden and associates30 described the first PT, using a slotted needle to guide a cutting trocar into the trachea. This technique was abandoned after fatalities resulted from trocar lacerations of vital structures adjacent to the airway.31,32 In 1969 Toye and Weinstein33 described a modified Seldinger technique in which a splitting needle was inserted into the trachea, and through this a guidewire was placed. A single lead dilator was passed over the guidewire, the needle split away, and the tracheostomy tube placed. This technique had a 1% incidence of perioperative death and a 6% incidence of paratracheal insertion, which ultimately caused it also to be abandoned.34 In 1985, Ciaglia and colleagues,35 drawing from experience with cricothyroidotomy, described a true Seldinger technique for quick and easy bedside tracheostomy: percutaneous dilational tracheostomy (PDT). In this technique, multiple curved dilators of gradually increasing size are placed over a guidewire, creating an opening for a tracheostomy tube. Subsequently, several different versions of PT have been described. In 1988, Hazard and associates36 had few complications using three straight dilators instead of Ciaglia’s seven curved dilators. In 1989, Schachner and colleagues37 reported a dilating forceps technique (Rapitrac), which is no longer available for use because of its high incidence of complications. In 1990, Griggs and coworkers38 described passing a blunt-tipped modified Kelly forceps over a guidewire to allow dilation of an aperture adequate to place a tracheostomy tube (guidewire dilator forceps [GWDF] technique). This technique is similar to the Rapitrac, but with a lower incidence of complications. In 1999, Ciaglia developed a soft-tipped, tapered dilator (Blue Rhino, Cook Critical Care, Inc., Bloomington, IN) that was used to create a stoma via a single-step dilation.39 This approach replaced the multiple dilations necessary in Ciaglia’s original kit, theoretically reducing complications and the time necessary to perform PDT. Fantoni’s translaryngeal tracheotomy technique was introduced in 1993 and modified in 1996,40 in which a guidewire is directed in retrograde fashion from the trachea to the mouth over which a cuffed cone-cannula is placed. The cannula is drawn through the neck, and a cuffed tube is placed in the trachea over the cannula as it is removed. The PercuTwist, developed in 2002, uses a dilator with a threaded screw to allow the insertion of a 9-mm tracheostomy tube.41 The latest modification of the Ciaglia technique of PDT was developed in 2005 and uses balloon dilatation to create the stoma (Blue Dolphin, Cook Critical Care, Inc., Bloomington, IN).42,43 In critical care medicine, regardless of the patient’s diagnosis, there are four indications for placement of an artificial airway, which can be either an endotracheal or tracheostomy tube: (1) relieving airway obstruction, (2) providing mechanical ventilation (MV), (3) preventing aspiration in the unprotected airway, and (4) facilitating tracheobronchial toilet.44,45 Although tracheostomy was considered the emergency airway of choice in the past, now endotracheal intubation is preferred for initial airway management. This is because more practitioners are familiar with endotracheal intubation (which requires less specialized equipment and training), and studies indicate that, in the emergency setting, endotracheal intubation has fewer life-threatening complications46–48 such as bleeding and pneumothorax, which are extremely rare. Independent of esophageal placement, mortality rate from endotracheal intubation is 0.05%, but ranges from 1% to 2% for emergency tracheostomy.29 Tracheostomy should be considered an elective or semielective procedure when the airway is already secured. Occasionally, endotracheal intubation under direct laryngoscopic visualization is not the initial airway management of choice because of massive facial trauma, tracheal obstruction, or anomalous anatomy.49,50 In such cases, endotracheal intubation sometimes can be facilitated through fiberoptic bronchoscopy.51 When intubation is impossible even with bronchoscopy, or when bronchoscopy is unavailable, the preferred emergency airway procedure is cricothyroidotomy.52,53 The primary role of tracheostomy is long-term airway care for patients who initially were treated with ETs or cricothyroidotomies, although reports of PT performed in an emergency setting have been published.54–57 The question as to when to replace an ET with a tracheostomy tube is a subject of much discussion, remaining highly controversial independent of technique. Beatrous44 stated in 1968 that, “Timing is an aspect of tracheostomy that deserves much more emphasis than it is apparently receiving. Delay defeats the purpose of the operation.” The debate as to when to replace an ET with a tracheostomy tube centers on the advantages and disadvantages of the tracheostomy tube, as listed in Table 14.1.58–126 Unfortunately there are very few definite studies confirming or refuting these advantages. Table 14.1 Advantages and Disadvantages of Tracheostomy *Improved survival after tracheostomy has not been demonstrated in all studies (see text). The decision as to when to perform a tracheostomy is more often based on personal preference of the treating physician than on data. For patients with an airway obstruction that cannot be relieved, tracheostomy is the treatment of choice because it is the only airway that can safely remain long term.127,128 In most other situations the decision is not as straightforward as ultimately only 5% to 11% of patients on MV require placement of a tracheostomy tube. Nevertheless, those patients account for 26% of all ventilator days and 14% of hospital days.101,125,129 Although the need for tracheostomy sometimes can be predicted early in the ICU course (e.g., neurologic ICU patients with infratentorial lesions who have brainstem dysfunction130 or a low Glasgow Coma Scale score90,99), in most situations this is not the case. Finding early clinical predictors that would identify patients who require a tracheostomy is most problematic in patients receiving MV.131–134 Many studies from the early and mid-1990s attempted to establish criteria to satisfy the recommendations of the 1989 Consensus Conference on Artificial Airways in Patients Receiving Mechanical Ventilation, which stated that “the decision to convert to tracheotomy should be made as early as possible . . . to minimize the duration of translaryngeal intubation. Once the decision is made, the procedure should be done without undue delay.”135 Unfortunately, good studies to determine optimal timing of tracheostomy are difficult to perform. Existing studies tend to be either retrospective reviews, which often include a large number of patients but are prone to bias, or randomized, controlled trials that are generally underpowered owing to the large number of patients required to detect significant outcome benefits.129 Multiple publications address the question of timing of tracheostomy in a variety of patient populations.85–92,115–120,124,136–138 Most are retrospective reviews of ICU databases in which timing of tracheostomy is retrospectively correlated with multiple outcomes. In these studies, the definition of “early” ranges from 3 to 21 days, and “late” ranges from 7 to 28 days, making it hard to draw any firm conclusions. It seems clear from these studies, however, that the procedural and short-term complications of tracheostomy are low, and that in general the outcome of patients with tracheostomy is at least no worse than that of patients managed with prolonged translaryngeal endotracheal intubation. Some studies show a decrease in mortality rate in patients with tracheostomy.100,101,120,125 However, one has to question if this difference is partly explained by the fact that patients with anticipated high mortality risk are not offered a tracheostomy. A frequent finding is that placement of a tracheostomy reduces time on MV,86,87,89–92,101,116,118,119,121 but even this is not consistent. Other studies have shown no change85,115,138 or even markedly prolonged times100 on MV. This may reflect either a less aggressive approach to weaning after a potentially permanent airway has been placed or a selection bias reflecting higher early mortality rate in the patients not receiving a tracheostomy. Randomized controlled trials of early tracheostomy versus late tracheostomy or prolonged translaryngeal intubation are rare. In 2004, Rumbak and associates87 showed that early tracheostomy in critically ill medical patients decreased time on MV, ICU length of stay, and ventilator-associated pneumonia. Additionally, early tracheostomy decreased mortality rate by 50% despite well-matched baseline characteristics. They also evaluated the patients for the most feared complication of tracheostomy, tracheal stenosis, during the hospital course and at 10 weeks. No significant differences in the incidence or severity of stenosis were seen, although there was a trend favoring early tracheostomy.87 These findings conflict with the findings of several other randomized controlled trials. A trial by Sugerman and coworkers72 showed no differences in any of the measured outcomes (ICU length of stay, death, and pneumonia). This study was significantly limited by incomplete data collection and physician bias. Another randomized controlled trial in trauma patients by Barquist and associates was prematurely terminated after the first interim analysis with only 60 patients enrolled due to lack of differences in the primary outcomes; ICU length of stay, ventilator-associated pneumonia, and ventilator days.136 A well-designed trial in France looked at multiple end points including death, pneumonia, duration of MV, complications, sedation requirements, and subjective patient comfort. There were no differences in any of the parameters, except in late laryngeal complications and patient discomfort. All patients who had an endotracheal as well as a tracheostomy tube during the course of their illness favored tracheostomy. Unfortunately, only 10% to 20% of eligible patients were actually enrolled in the trial and the study was grossly underpowered to detect any significant differences.115 Finally, a study assessing the impact of early tracheostomy (6-8 days) on the risk of developing ventilator-associated pneumonia showed a statistically insignificant trend (p = 0.07) toward reduction in the incidence of pneumonia.120 Another large trial showed no benefit of early tracheostomy except reduced use of sedatives.123 Interpreting length of ICU and hospitalization data from these trials requires knowledge of post-tracheostomy disposition, which may vary depending on the country in which the study was performed. Specifically, the availability of long-term ventilator facilities might not be apparent. An older trial in the 1970s by Stauffer and colleagues,69 which is frequently quoted as an argument for prolonged translaryngeal intubation, found significantly more complications that were judged more severe in patients who underwent tracheostomy compared to patients with translaryngeal intubation. Most notably, the incidence of tracheal stenosis after tracheostomy was significantly higher (65% versus 19%). Nonetheless, they also found that patients treated with prolonged translaryngeal intubation followed by tracheostomy had significantly more laryngeal injury and an increased frequency of tracheal stenosis compared with patients who underwent tracheostomy after a short period of translaryngeal intubation.69 Conclusions from all these studies indicate that a tracheostomy should be offered only to patients anticipated to survive who require secure, long-term airway access. The timing of tracheostomy tube placement should be individualized and depends on three factors: (1) underlying disease, (2) indication for artificial airway, and (3) expected ultimate outcome. An article published in 2012 found that the hospital mortality rate of patients undergoing PDT was 30% with 14-day and 6-month mortality rates of 11% and 40%, respectively.122 Predictors of poor short-term outcome were found to be older age, diagnosis of malignancy, cardiogenic shock, and presence of a ventricular assist device. A realistic discussion of prognosis therefore should be part of the informed consent prior to placement of a tracheostomy. Despite several negative trials that were generally underpowered and with the emergence of bedside PDT, a recommendation for earlier tracheostomy still seems appropriate as the early complication rate in experienced hands is low and is likely outweighed by the probable benefits, especially patient comfort. Figures 14.1 through 14.3 provide suggested algorithms regarding the timing of tracheostomy placement based on whether the patient’s pathologic condition is primarily upper airway obstruction, neuromuscular, or pulmonary. In patients who have upper airway obstruction, the timing of tracheostomy depends on the likelihood of rapid resolution of the obstruction and whether surgical intervention is required (see Fig. 14.1). Many ICU patients intubated because of stupor, coma, or neuromuscular weakness (see Fig. 14.2) are susceptible to aspiration, as they may have laryngeal dysfunction and may not be able to generate an effective cough.139 In most patients, the duration of the pathologic condition is predictable, either short (e.g., drug overdose) or long (e.g., large stroke, amyotrophic lateral sclerosis).140–145 In some cases of postoperative weakness, cerebrovascular accident, and peripheral neuromuscular weakness (e.g., Guillain-Barré syndrome, myasthenia gravis), the course is uncertain.139,146–149 A second factor involved in the decision to perform tracheostomy is the coexistence of pulmonary complications, such as atelectasis, pneumonia, or aspiration (see Fig. 14.3). The spectrum of diseases for patients intubated because they require MV is so broad that firm rules regarding tracheostomy have not been established. The approach to the timing of tracheostomy in these situations is difficult because the duration of ventilatory support is frequently hard to predict. This is demonstrated in some randomized studies comparing early and late tracheostomy in which 50% to 75% of patients randomized to delayed tracheostomy did not undergo this procedure because of either death or extubation.115,120,150 Despite this and multiple studies not supporting survival benefits or faster liberation from ventilator support but with data supporting improved patient well-being, it is advisable to consider early tracheostomy once the patient has stabilized, if the patient is expected to survive and needs a long-term airway, and a tracheostomy can be safely performed. However, a recently published paper followed 73 ICU patients who received a tracheostomy and were transferred to a floor experienced in the care of patients with tracheostomies.126 Patients who were not decannulated in the ICU had a significantly higher mortality rate compared to the patients who were decannulated prior to transfer (11% versus 26%). The only other factors associated with increased mortality rate were the presence of tenacious sputum at ICU discharge and a body mass index (BMI) greater than 30 kg/m2. “The cause of in-ward deaths was cardiopulmonary arrest in 33% of ICU-decannulated patients, versus 90% of those discharged with a cannula in place (p = 0.08), most of which occurred overnight.” The authors feel the most likely cause of death was respiratory arrest that was likely cannula related, suggesting suboptimal monitoring and care for nondecannulated patients after ICU discharge. This study, though very concerning, was a single-center, prospective, observational trial. Therefore, this finding may reflect an institutional problem rather than a problem inherent to the procedure. Nevertheless, this issue needs further investigation. Throughout the history of tracheostomy, complications have been the most scrutinized aspect. Complications have been categorized as occurring during performance of the tracheostomy (procedural), while the tube is in place (in situ), or after decannulation.29,102,106 Older studies report a major complication rate of approximately 15% (range, 6% to 66%), with a mortality rate of approximately 1.5% (range, 0% to 5%).29,47,48,69,71,102–107,151 More recent studies show generally lower complication rates ranging from 1.4% to 27%, and essentially negligible mortality rate.152–158 A recent multicenter review showed that major procedural complications are rare and are only dependent on operator experience, but not surgical technique or location of the procedure.157 The most common serious procedural complications are pneumothorax (0.9% to 5%) and severe hemorrhage (5%).44,47,48,71,103,105,151 Pneumothorax usually results from violation of the pleural space as it ascends into the neck. Operative hemorrhage is usually venous, originating from the anterior jugular venous system, the thyroid gland isthmus, and very rarely may be arterial from an aberrant vessel. Most of the time bleeding can be controlled by local measures alone, but occasionally will require surgical intervention for hemostasis.156 Other serious but very rare complications (<1%) include tube misplacement, tracheoesophageal perforation, aspiration, thyroid laceration, recurrent laryngeal nerve damage, and cardiopulmonary arrest.29,48,151,152,156 Less serious complications are subcutaneous and mediastinal emphysema, clinically insignificant hypotension, and desaturation.152–154,157 After a tracheostomy tube is placed, it is susceptible to obstruction and displacement, which may be potentially life-threatening, especially if a problem occurs before a secure tract has been formed and is not promptly recognized.29,48,106 Tube displacement may result from poor tracheostomy tube selection, excessive patient motion, or careless reinsertion after dislodgement. Obstruction is often caused by tenacious secretions, but can also be caused by positioning of the tracheostomy tube against the wall after poor sizing of the tracheostomy. Use of a double-cannula tube with a disposable inner cannula protects against occlusion by encrusted secretions,8,159 though this may delay successful weaning because of the increased outer diameter of the tube.160 Increasing peak airway pressure, increased resistance during manual bag ventilation, or difficulty passing a suction catheter may indicate tube obstruction or displacement. Providing adequate humidity, adhering to suctioning and tracheostomy care protocols, and minimizing tube manipulation help avert this.48,106,151 Another common in situ complication is stomal infection, which occurs in 36% of all tracheostomies.69 Trivial stomal infection can be treated topically.48 Necrotizing wound infection can occur, and may extend into the mediastinum, causing life-threatening sepsis.154,161 Many patients will have bacterial colonization of the tracheobronchial tree, especially with resistant gram-negative organisms (50% to 66%) making them more susceptible to develop nosocomial pneumonia.29,103,127,162
Tracheostomy
History
The Artificial Airway
Timing of Tracheostomy
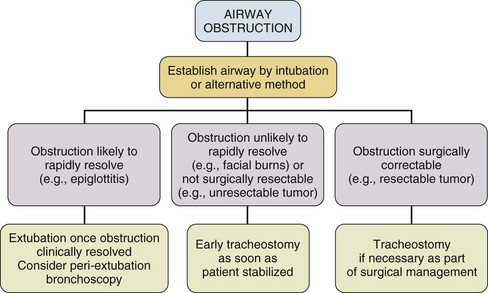
Upper Airway Obstruction
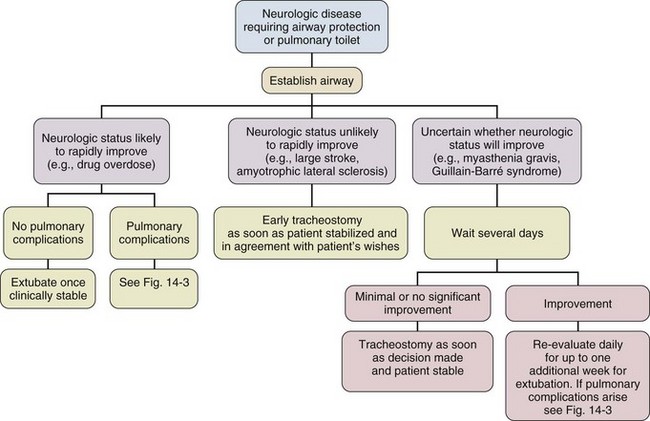
Neuromuscular Conditions
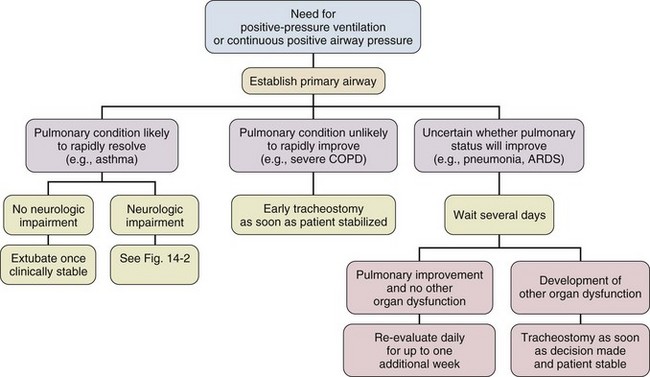
Pulmonary Conditions
Complications of Tracheostomy
Procedural Complications
In Situ Complications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tracheostomy