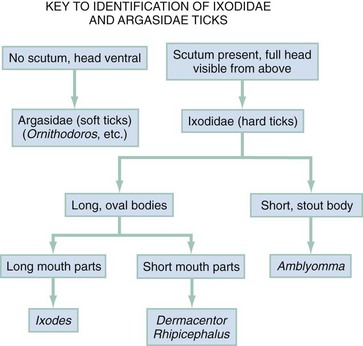
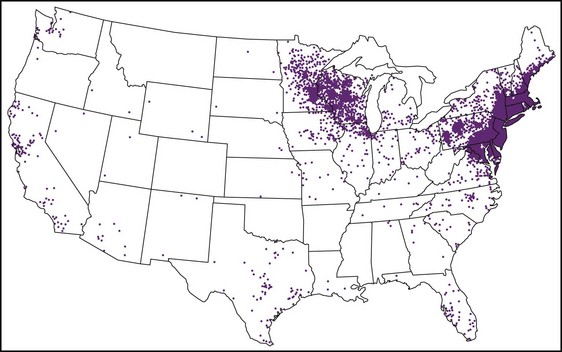
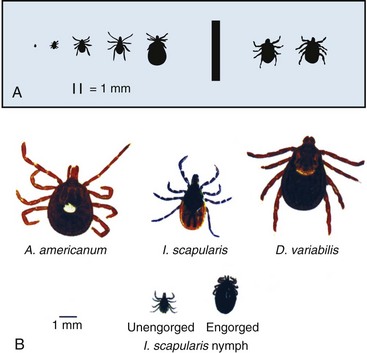
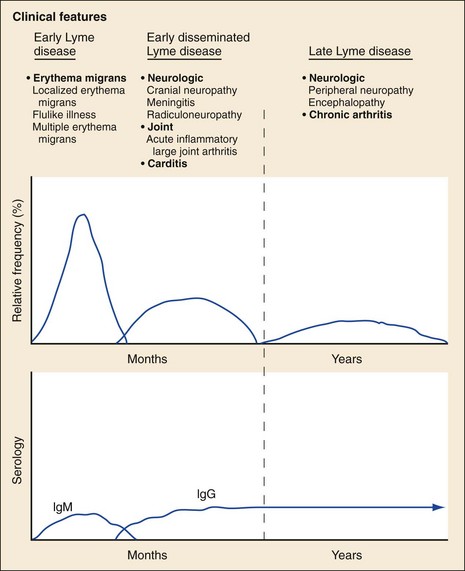
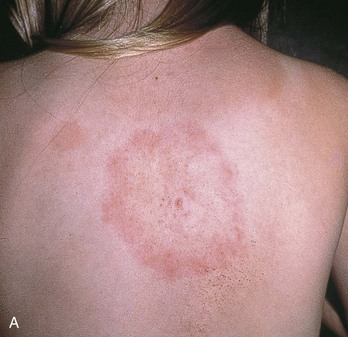
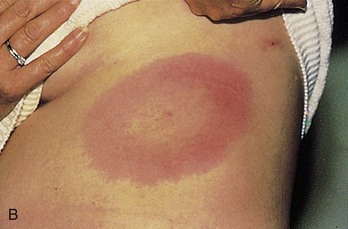
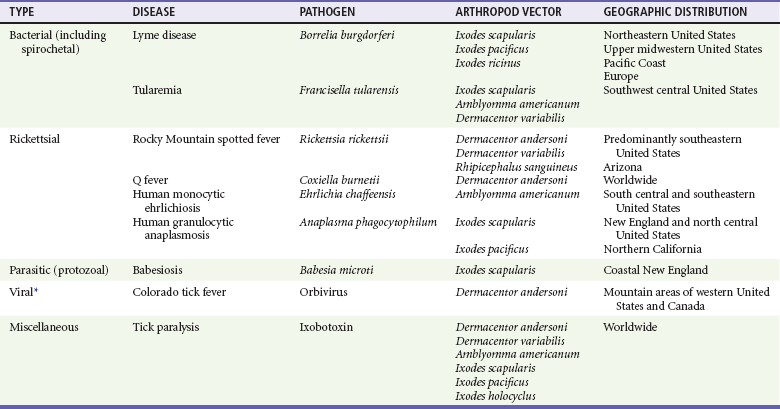
Chapter 134 Ticks are hematophagous parasites of humans and animals, distributed worldwide. They transmit rickettsial, bacterial, spirochetal, viral, and protozoal diseases and cause disease by means of their own toxins (Table 134-1). As vectors of human disease, ticks rank second in importance only to mosquitoes. People who travel during the summer months may return from endemic areas with tick-borne disease. In addition, reports of infection acquired within urban areas emphasize the need to consider tick-borne illness even in the absence of a history of travel to high-risk areas. Tularemia (category A) and Q fever (category B) are now considered by the Centers for Disease Control and Prevention (CDC) to be significant threats during biologic warfare. For this reason, research involving ticks and their diseases has become increasingly important. Table 134-1 *Many other viruses are transmitted to humans by ticks. In the United States, only Colorado tick fever occurs with any significant frequency. Reports on ticks, their feeding habits, and their possible relation to disease can be found from early history. Pliny (CE 77), in Historia Naturalis, referred to “an animal living on blood with its head always fixed and swelling, being one of the animals which has no exit [anus] for its food, it bursts with over-repletion and dies from actual nourishment.”1 Tick-borne illness was first recognized on the North American continent by Native Americans. According to legend, Shoshone men avoided the “evil spirits” that caused illness by sending only women into certain areas of the Rocky Mountain region known to be especially hazardous. The etiologic association of the tick vector with Rocky Mountain spotted fever (RMSF) was noted by missionaries and by early settlers, who named the affliction tick fever. Physicians in Idaho and Montana recorded the classic clinical descriptions of the disease in 1899. Ticks are arthropods but not insects. They have eight legs instead of six and generally two fusing body parts—a capitulum (head) and an opisthosoma (abdomen)—instead of three. Identification of an arthropod as a tick and subsequent categorization into family and some genera are not difficult (Figs. 134-1 and 134-2). Speciation requires a trained acarologist. However, tick identification has limited importance in clinical decision-making. Color, which varies seasonally, and size, which varies by amount of blood ingested at the time of presentation, are unreliable criteria for identification purposes. Lyme disease, the most common vector-borne disease in the United States, is a tick-borne illness caused by the spirochete Borrelia burgdorferi. The story of Lyme disease began in 1975, when health officials at Connecticut’s State Department of Health and physicians at Yale University were alerted by two skeptical mothers to an unusually large number of cases of apparent juvenile rheumatoid arthritis occurring in their small coastal community of Old Lyme, Connecticut. Investigation led to the description of a “new” entity called Lyme arthritis.2 Lyme disease occurs worldwide and has been reported on every continent except Antarctica.3 It now accounts for more than 95% of all reported cases of vector-borne illness in the United States. The actual overall incidence of Lyme disease is unknown because many cases go unreported. Lyme disease occurs in people of all ages but is more common in children younger than 15 years and in adults 30 to 60 years of age.4 Persons at greatest risk live or vacation in endemic areas. In the United States, three distinct endemic foci are recognized: the northeastern coastal, mid-Atlantic, and north central states. During 2000, a total of 17,730 cases of Lyme disease were reported from 44 states and the District of Columbia. Twelve states—Connecticut, Rhode Island, New Jersey, New York, Delaware, Pennsylvania, Massachusetts, Maryland, Wisconsin, Minnesota, New Hampshire, and Vermont—accounted for 95% of cases reported in the nation (Fig. 134-3).4 Although all stages of the tick may feed on humans, the nymph is primarily responsible for the transmission of Lyme disease. It is not surprising that more than two thirds of patients with Lyme disease do not recall a tick bite, in view of the small size (1-2 mm) of nymphs (Fig. 134-4). The nymph feeds in the spring and summer, which correlates with a peak incidence of early Lyme disease occurring between May and August. In addition, recreational and occupational exposure is greatest during this time. Later manifestations of Lyme disease may appear throughout the year. Lyme disease, a multisystem disorder, can be classified into three stages: early localized, early disseminated, and late disease. Virtually any clinical feature may occur alone or recur at intervals, and some patients who had no early symptoms may have late symptoms. The disorder usually begins with a rash and associated constitutional signs and symptoms, suggesting a “viral syndrome” (early Lyme disease). Neurologic, joint, or cardiac manifestations may emerge weeks to months later (early disseminated Lyme disease), and chronic arthritic and neurologic abnormalities may appear weeks to years later (late Lyme disease). The time course for the clinical features of untreated Lyme disease is illustrated in Figure 134-5. Ticks may attach to human hosts at the initial point of contact (generally around ankle level) or move about until they encounter an obstruction. The groin, popliteal fossae, gluteal folds, axillary folds, and earlobes are common sites of attachment. After transmission of B. burgdorferi through a tick bite, the initial site of infection is the skin at the site of the bite. After an incubation period of approximately 1 week (range, 1-36 days), the spirochetes cause a gradually spreading localized infection in skin and a resultant skin lesion, erythema migrans. Erythema migrans is the most characteristic clinical manifestation of Lyme disease and is recognized in 90% or more of patients. Erythema migrans may go unnoticed if the entire skin surface is not examined.5 The characteristic rash begins at the site of the tick bite with an erythematous papule or macule. The lesion expands gradually (1-2 cm/day, a rate of expansion slower than that of cellulitis). The patch of erythema may be confluent or may have bands of normal-appearing skin. Central clearing may occur but is not an invariable feature. The lesion borders usually are flat but may be raised. The lesions generally are sharply demarcated and blanch with pressure. Most lesions are oval or round, but triangular and elongated patches may occur. In patients presenting 1 to 7 days after the appearance of lesions, the average lesion size is approximately 8 by 10 cm (range, 2 by 3 cm to 25 by 25 cm). In some cases, the center of some early lesions becomes red and indurated or vesicular and necrotic. The lesion is warm to the touch and may be described by the patient as nontender to minimally tender (Fig. 134-6). Constitutional signs and symptoms commonly appear in early Lyme disease (Table 134-2). Malaise, fatigue, and lethargy are most common (seen in approximately 80% of patients) and may be severe. Fever typically is low grade and intermittent. Lymphadenopathy usually is regional in the distribution of erythema migrans or may be generalized; splenomegaly may occur. Musculoskeletal complaints, such as arthralgias and myalgias, are common, and the discomfort typically is short-lived and migratory, sometimes lasting only hours in one location. Frank arthritis may occur at this stage but is rare. Table 134-2 Early Clinical Manifestations of Lyme Disease *Required for inclusion in this study. From Steere AC, et al: The early clinical manifestations of Lyme disease. Ann Intern Med 99:76, 1983. The incidence of Lyme disease without erythema migrans appears to be approximately 10%.5 Because of the variety of nonspecific signs and symptoms at this stage, in the absence of the characteristic rash or history of tick bite, early Lyme disease may be easily confused with a viral or collagen vascular disease. The intermittent and rapidly changing nature of the early signs and symptoms of Lyme disease may be a helpful distinguishing feature, especially in a patient from an endemic area. In untreated disease, early symptoms usually last for several weeks but may persist for months. Neurologic Manifestations.: A relatively symptom-free interval usually occurs between early and disseminated infection; however, neurologic signs and symptoms may be the presenting manifestations of Lyme disease or may overlap with early or late manifestations. Beginning at an average of 4 weeks (range, 0-10 weeks) after the onset of erythema migrans, neurologic involvement occurs in approximately 15% of untreated patients. The most common neurologic manifestation of Lyme disease is a fluctuating meningoencephalitis with superimposed symptoms of cranial neuropathy, peripheral neuropathy, or radiculopathy. A triad of meningitis, cranial neuropathies (usually Bell’s palsy), and radiculopathy has been described, but each entity may occur alone. Headache of variable intensity usually is present; other signs and symptoms of a mild meningoencephalitis may be noted, including lethargy or irritability, sleep disturbances, poor concentration, and memory loss. At this point, the disease often is misdiagnosed as viral meningitis. As in early disease, Kernig’s and Brudzinski’s signs are absent and computed tomography findings are normal. Unlike in early disease, however, findings on CSF examination often are abnormal, with a lymphocytic pleocytosis and moderately elevated protein level. CSF glucose concentration usually is normal. Intrathecal B. burgdorferi antibody (usually IgG or IgA) is present in 80 to 90% of patients. CSF polymerase chain reaction (PCR) assay results are positive in less than half of patients,6 probably reflecting the low number of organisms usually present in spinal fluid. Routine testing of CSF by PCR is not recommended. Cardiac Manifestations.: Cardiac involvement in Lyme disease is uncommon. Estimates of the incidence of carditis in untreated patients who have Lyme disease range from 4 to 10%. The average time from initial illness to the development of carditis typically is 3 to 5 weeks (range, 4 days to 7 months). Direct myocardial invasion has been demonstrated with endomyocardial biopsy. Electrophysiologic testing has demonstrated widespread involvement of the conduction system. The most common cardiac manifestation of Lyme disease is atrioventricular (AV) block, although conduction defects may involve any level of the conducting system. Myopericarditis, tachydysrhythmias, and ventricular impairment occur less often. In a review of 105 reported cases of Lyme carditis, 49% of cases were third-degree AV block, 16% were second-degree, and 12% were first-degree.7 The degree of AV block seen in a specific patient may fluctuate rapidly. Arthritis.: Although it is classically considered a sign of late Lyme disease, acute arthritis may begin during the acute disseminated stage. Monarticular or oligoarticular arthritis, primarily affecting large joints, especially the knee, may develop weeks to months after the onset of initial illness. In an early study of the natural history of Lyme arthritis, approximately 50% of untreated patients experienced one episode or multiple intermittent attacks of arthritis. Acute arthritis typically is monarticular, with involvement of only one knee. The shoulder, elbow, temporomandibular joint, ankle, wrist, hip, and small joints of the hands and feet are involved less commonly. Episodes of arthritis typically are brief (lasting weeks to months) and separated by variable periods of remission. A pattern of exacerbation and remission of arthritis may extend for several years, with a gradual tendency toward less frequent and less severe occurrences. The spontaneous long-term remission rate approximates 10 to 20% annually in untreated patients. However, patients commonly have episodes of periarticular involvement, arthralgias, or fatigue interspersed between attacks of frank arthritis. During the second or third year of illness, attacks of joint swelling sometimes become longer in duration, lasting months rather than weeks. Chronic arthritis eventually develops in approximately 10% of patients.8 The most common late neurologic manifestation of Lyme disease is a chronic encephalopathy that is manifested as a mild to moderately severe impairment of memory and learning. Hypersomnolence and mild psychiatric disturbances (depression, irritability, or paranoia) also may develop.6 Peripheral nervous system manifestations often are seen in late disease, with involvement of cranial nerves, spinal roots, spinal plexuses, and peripheral nerves. A predominantly sensory polyradiculoneuropathy that is manifested as either radicular pain or distal paresthesia is common. Significant overlap occurs with early symptoms. Less commonly, a demyelinating condition resembling multiple sclerosis may appear in late disease. Symptoms are variable and, as in multiple sclerosis, may undergo exacerbations and remissions. Computed tomography and magnetic resonance imaging may reveal multiple white matter lesions.9 Results of routine laboratory studies are nonspecific, and such studies generally are not helpful in diagnosis of Lyme disease. Abnormalities may include an elevated erythrocyte sedimentation rate, mild anemia, total white blood cell count in the normal range with a decreased absolute lymphocyte count, microhematuria, proteinuria, and increased alanine transferase level. Cultures of blood, tissue, and body fluids (including CSF and synovial fluid) for B. burgdorferi and direct visualization techniques are difficult to perform properly and have such a low yield that they are not clinically useful.10 IgM and IgG immunoblots should be obtained if early disease is suspected. If late disease is suspected, IgG Western blot alone should be obtained. Criteria for positive Western immunoblotting (requiring the presence of bands at particular locations) have been adopted by the CDC.10 IgG (and occasionally IgM) antibody may persist for several years after adequate treatment and symptom resolution. Persistent seropositivity is not diagnostic of ongoing infection. Even an IgM response cannot be interpreted as a demonstration of recent infection or reinfection unless the appropriate clinical characteristics are present. IgG antibody developed after natural infection does not always confer immunity against future infection by B. burgdorferi. Patients who are treated for erythema migrans may become reinfected; patients with Lyme arthritis, however, usually have high antibody titers to many spirochetal proteins and seem not to become reinfected.11 False-positive ELISA results are common. Serologic cross-reactivity can occur between B. burgdorferi and other spirochetes, most notably Treponema pallidum. False-positive results for Lyme disease also can occur with relapsing fever, gingivitis, leptospirosis, enteroviral and other viral illnesses, rickettsial diseases, autoimmune diseases, malaria, and subacute bacterial endocarditis. In addition, it is estimated that up to 5% of the normal population will “test positive” for Lyme disease by ELISA. Bayes’ theorem states that if the pretest likelihood of the disease is low, the positive predictive value is low: a positive test result is more likely to be a false-positive result. For this reason, screening serologic tests are not indicated in the absence of objective clinical evidence of Lyme disease.12 Patients suspected of having acute Lyme neuroborreliosis should be evaluated with serologic tests and routine CSF examination. Paired serum and CSF samples should be obtained to evaluate for intrathecal production of antibody, although most patients with neuroborreliosis have positive results on serum serologic testing, thereby making additional laboratory confirmation with CSF serology unnecessary.12 PCR has low sensitivity when it is performed on CSF and is not routinely recommended.6 PCR assay is superior to culture for the detection of B. burgdorferi in synovial fluid and has a sensitivity of 73% and specificity of 99% in untreated Lyme arthritis.13 Prompt treatment of early disease can shorten the duration of symptoms and prevent progression to later stages of disease. Most of the various manifestations of Lyme disease can be treated successfully with oral antibiotic therapy, with the exception of neurologic abnormalities, which usually require intravenous therapy. Treatment of Lyme disease is summarized in Table 134-3. Table 134-3 *Pediatric dosage should not exceed adult dosage. †Tetracycline, 250 to 500 mg PO qid, may be substituted for doxycycline. Neither doxycycline nor any other tetracycline should be used for children younger than 8 years or for pregnant or lactating women. ‡Regimens for radiculoneuropathy, peripheral neuropathy, and encephalitis are the same as those for meningitis. §Oral regimens are reserved for mild cardiac involvement (see text). Modified from Treatment of Lyme disease: New drugs for allergic conjunctivitis. Med Lett Drugs Ther 42:37, 2000; and Wormser GP, et al: The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089, 2006. The drug of choice for men, nonpregnant and nonlactating women, and children older than 8 years is doxycycline, 100 mg twice daily for 3 weeks.3 An advantage of doxycycline is that it also is effective for treatment of human granulocytic anaplasmosis, which is transmitted by the same tick that transmits Lyme disease. Pregnant or lactating women and children younger than 8 years should receive amoxicillin, 500 mg orally (20 to 40 mg/kg per day in three doses for children). Cefuroxime axetil has been shown to be as effective as doxycycline and may be used in children of any age, but cephalexin is ineffective in Lyme disease.14 Macrolide antibiotics are not recommended as first-line agents for therapy for early Lyme disease.14 They should be reserved for patients who cannot tolerate doxycycline, amoxicillin, and cefuroxime axetil. Macrolide regimens for adults include azithromycin, 500 mg orally daily for 7 to 10 days3; erythromycin, 500 mg orally four times daily for 14 to 21 days; and clarithromycin, 500 mg orally twice daily for 14 to 21 days. Neurologic Disease.: For patients with relatively mild symptoms (e.g., solitary facial nerve palsy with normal findings on CSF examination), doxycycline or amoxicillin can be used in the same dosage as for early disease, but the duration of therapy should be extended to 28 days. The use of prednisone for facial nerve palsy from Lyme disease has been suggested but is not currently recommended. For patients with other objective neurologic abnormalities (e.g., meningitis or encephalitis, peripheral neuropathies, cranial neuritis other than facial nerve palsy) or evidence of the spirochete in the CSF, parenteral antibiotic therapy is required. Ceftriaxone, 2 g/day intravenously for 14 days (75 to 100 mg/kg/day for pediatric patients), or penicillin G, 18 to 24 million units daily intravenously for 10 to 14 days, may be used.14 Ceftriaxone may be more effective than penicillin, and many experts recommend longer courses (e.g., up to 4 weeks). In cases of penicillin or cephalosporin allergy, oral doxycycline for 28 days may be used. Cardiac Disease.: Patients with mild cardiac conduction system involvement (first-degree AV block with a PR interval of less than 0.30 second) and no other significant symptoms usually can be treated safely on an outpatient basis with oral doxycycline or amoxicillin for 21 to 30 days.14 Patients with higher degrees of AV block, including first-degree block with a PR interval of more than 0.30 second or evidence of global ventricular impairment, should be hospitalized for cardiac monitoring and treatment with parenteral antibiotics. Either penicillin G, 18 to 24 million units intravenously, or ceftriaxone, 2 g daily for 21 days (50 to 80 mg/kg/day for children), may be used. Arthritis.: In established Lyme arthritis, the response to antibiotic therapy may be delayed for several weeks or months.3 Thirty-day oral regimens, such as doxycycline, 100 mg orally twice daily, or amoxicillin, 500 mg three times daily, usually are effective and, for reasons of cost and convenience, may be selected as first-line therapy given on an outpatient basis before use of parenteral antibiotic therapy is considered.14 Persistent or recurrent joint swelling after recommended courses of antibiotic therapy can be treated with another 4-week course of oral antibiotics or with a 2- to 4-week course of intravenous ceftriaxone.3 A small percentage of patients with Lyme arthritis, particularly those with HLA-DR4 specificity or antibody reactivity with OspA, may have persistent joint inflammation despite treatment with either oral or intravenous antibiotics. Such patients often do not respond to any antibiotic therapy and may require arthroscopic synovectomy. Neurologic Disease.: Patients with late neurologic disease affecting the central or peripheral nervous system should be treated with ceftriaxone (2 g once a day intravenously for 2 to 4 weeks). Alternative parenteral therapy may include cefotaxime (2 g intravenously every 8 hours) or penicillin G (18 million to 24 million units daily, given in divided doses every 4 hours). Response to treatment is usually slow and may be incomplete. Lyme disease contracted during pregnancy can be treated and cured. Treatment of pregnant patients can be identical to that of nonpregnant patients with the same disease manifestations, except that doxycycline should be avoided.3 Most women give birth to normal infants despite documented Lyme borreliosis during their pregnancies. No vaccine against Lyme disease is currently available in the United States. The LYMErix vaccine (SmithKline Pharmaceuticals, Philadelphia), initially licensed in 1999, was withdrawn from the market in 2002. The vaccine, directed against the outer surface protein A of B. burgdorferi (OspA), was apparently safe and efficacious but required multiple and repeated doses for optimal protection. Ongoing questions about its safety and cost-effectiveness dampened demand for the vaccine.15 Although previous expert consensus has recommended that persons bitten by deer ticks (I. scapularis) should not routinely receive antimicrobial chemoprophylaxis,3 this recommendation should be modified in accordance with the findings of a well-designed trial in which a single 200-mg dose of doxycycline given within 72 hours after tick bite effectively prevented Lyme disease.15 A single 200-mg dose of doxycycline should be considered for adult patients and children 8 years of age and older (4 mg/kg, up to a maximum dose of 200 mg) when all of the following criteria are met: (1) the tick is an adult or nymphal I. scapularis, (2) the tick has been attached for 36 hours or more as indicated by certainty of the time of exposure or the degree of engorgement, (3) prophylaxis can be started within 72 hours after tick removal, (4) the local rate of infection of these ticks with B. burgdorferi is 20% or greater, and (5) doxycycline is not contraindicated. Infection rates of 20% or greater of ticks with B. burgdorferi generally are reported from highly endemic areas such as New England, parts of the mid-Atlantic region, and parts of Minnesota and Wisconsin. Most other areas of the United States do not have infection rates high enough to warrant prophylaxis.3 The efficacy of single-dose doxycycline in patients who present more than 72 hours after removal of a tick is unknown. In children, dosing and efficacy of prophylactic treatment have not been evaluated. The effectiveness of doxycycline for the prevention of other infections transmitted by I. scapularis ticks (e.g., babesiosis, human granulocytic anaplasmosis) is unknown and should not be assumed.15 Other antimicrobial agents that are effective for the treatment of Lyme disease (e.g., amoxicillin) and even other regimens of doxycycline (e.g., 100 mg twice daily) have unknown efficacy for Lyme disease prophylaxis. Tick-borne relapsing fever is maintained in an animal reservoir consisting primarily of wild rodents, including squirrels, mice, rats, chipmunks, and rabbits. It is found predominantly at altitudes of 2000 to 7000 feet in coniferous forest habitats.16 The tick vectors are argasids belonging to several species of the genus Ornithodoros, which routinely reside in the nests and burrows of their mammalian hosts. Ticks acquire the infection by feeding on a spirochetemic rodent. The borreliae remain viable in the ticks for several years and can be passed transovarially to the next generation; thus the tick is a major reservoir and vector. These soft ticks feed for brief periods (15-120 minutes), usually at night, and their painless bite generally is unnoticed by the sleeping victim. Transmission occurs by injection of infected saliva through the bite site or intact skin. Less common modes of transmission (e.g., by way of venipuncture equipment in injection drug users) have been reported. In the United States, relapsing fever occurs primarily in the western Mountain and Pacific states, including Montana, Wyoming, Nevada, Colorado, California, and Washington. Persons who come in contact with infected ticks from wild rodents are at greatest risk. Outbreaks have been reported among groups of persons sleeping overnight in hunting cabins inhabited by wild rodents.16,17 Clinical illness is manifested in two classic stages as each fever episode resolves. The first stage is called the “chill” phase (high fevers with reported temperatures of up to 106.7° F, mental status changes, tachycardia, and tachypnea), lasting approximately 30 minutes, followed by a “flush” phase (rapid temperature decrease, sweats, and hypotension), which can be confused with a Jarisch-Herxheimer reaction.18
Tick-Borne Illnesses
Perspective
Principles of Disease
Lyme Disease
Clinical Features
Early Lyme Disease
MANIFESTATION
NO. OF PATIENTS (%)
Signs
Erythema chronicum migrans*
314 (100)
Multiple annular lesions
150 (48)
Lymphadenopathy
Regional
128 (41)
Generalized
63 (20)
Pain on neck flexion
52 (17)
Malar rash
41 (13)
Erythematous throat
38 (12)
Conjunctivitis
35 (11)
Symptoms
Malaise, fatigue, lethargy
251 (80)
Headache
200 (64)
Fever and chills
185 (59)
Stiff neck
151 (48)
Arthralgias
150 (48)
Myalgias
135 (43)
Backache
81 (26)
Anorexia
73 (23)
Sore throat
53 (17)
Nausea
53 (17)
Dysesthesia
35 (11)
Vomiting
32 (10)
Acute Disseminated Infection
Late Lyme Disease
Diagnostic Strategies
Management
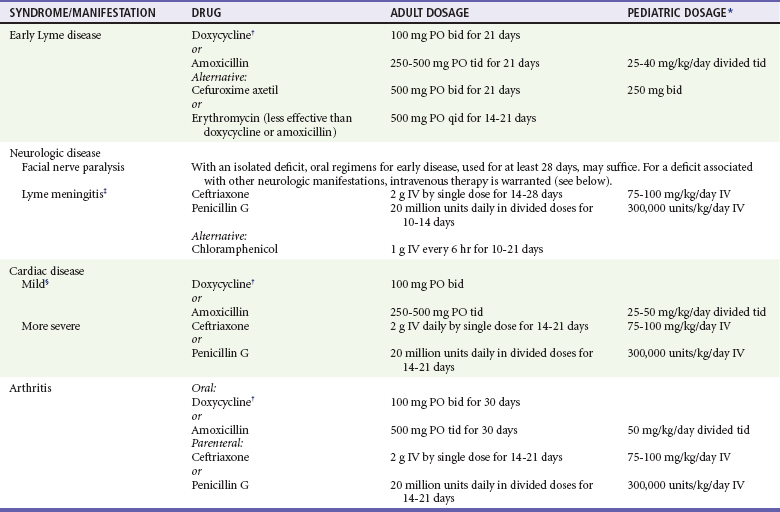
Early Disease
Early Disseminated Infection
Late Infection
Lyme Disease and Pregnancy
Vaccination
Prophylaxis and Asymptomatic Tick Bites
Relapsing Fever
Principles of Disease
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree