166 Thyroid Gland Disorders
 Normal Thyroid Hormone Economy
Normal Thyroid Hormone Economy
Regulation
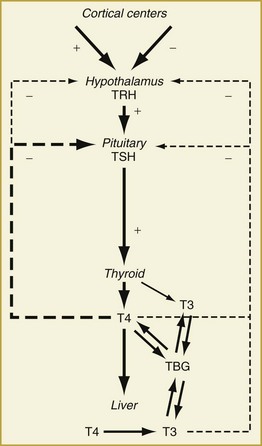
Synthesis and secretion of thyroid hormone is under the control of the anterior pituitary hormone, thyrotropin (or thyroid-stimulating hormone [TSH]). Following a classic negative feedback system, TSH secretion increases when serum thyroid hormone levels fall and decreases when they rise (Figure 166-1). TSH secretion is also under the regulation of the hypothalamic hormone, thyrotropin-releasing hormone (TRH). The negative feedback of thyroid hormone is targeted mainly at the pituitary level but likely affects TRH release from the hypothalamus as well. In addition, input from higher cortical centers can affect hypothalamic TRH secretion.
Under the influence of TSH, the thyroid gland synthesizes and releases thyroid hormone. Thyroxine (T4, 65% iodine by weight) is the principal secretory product of the thyroid gland, comprising about 90% of secreted thyroid hormone under normal conditions.1 Whereas T4 may have direct actions in some tissues, it primarily functions as a hormone precursor that is metabolized in peripheral tissues to the transcriptionally active 3,5,3′-triiodothyronine (T3, 59% iodine by weight).
Metabolic Pathways
The major pathway of metabolism of T4 is by sequential monodeiodination.2 At least three deiodinases, each with its unique expression in different organs, catalyze the deiodination reactions involved in the metabolism of T4. Removal of the 5′-, or outer ring, iodine by type I iodothyronine 5′-deiodinase (D1) or type II iodothyronine 5′-deiodinase (D2) is the “activating” metabolic pathway leading to formation of T3. Removal of the inner ring, or 5-, iodine by type III iodothyronine deiodinase D3 is the “inactivating” pathway producing the metabolically inactive hormone, 3,3′,5′-triiodothyronine (reverse T3, rT3). D1 is found most abundantly in the liver, kidneys, and thyroid. It is up-regulated in hyperthyroidism and down-regulated in hypothyroidism. D2 is found primarily in the brain, pituitary, and skeletal muscle and is down-regulated in hyperthyroidism and up-regulated in hypothyroidism. D3 is expressed primarily in the brain, in skin, and in placental and chorionic membranes. The actions of D3 also include inactivation of T3 to form T2, another inactive metabolite. Under normal conditions, about 41% of T4 is converted to T3, about 38% is converted to rT3, and about 21% is metabolized via other pathways, such as conjugation in the liver and excretion in bile.4,5
T3 is the metabolically active thyroid hormone and exerts its actions via binding to chromatin-bound nuclear receptors and regulating gene transcription in responsive tissues.3 Important in understanding the alterations in circulating thyroid hormone levels seen in critical illness is the fact that only around 10% of circulating T3 is secreted directly by the thyroid gland while more than 80% of T3 is derived from conversion of T4 in peripheral tissues.1,2 Thus, factors that affect peripheral T4-to-T3 conversion will have significant effects on circulating T3 levels. Serum levels of T3 are approximately 100-fold less than those of T4, and like T4, T3 is metabolized by deiodination to form diiodothyronine (T2) and by conjugation in the liver. The half-lives of circulating T4 and T3 are 5 to 8 days and 1.3 to 3 days, respectively.4
Serum Binding Proteins
Both T4 and T3 circulate in the serum as hormones bound to several proteins synthesized by the liver.5 Thyroid-binding globulin (TBG) is the predominant transport protein and binds roughly 80% of the circulating serum thyroid hormones. The affinity of T4 for TBG is about 10-fold greater than that of T3 and is part of the reason circulating T4 levels are higher than T3 levels. Other serum binding proteins include transthyretin,6 which binds some 15% of T4 but little if any T3, and albumin, which has a low affinity but a very large binding capacity for T4 and T3. Overall, 99.97% of circulating T4 and 99.7% of circulating T3 is bound to plasma proteins.
 Thyroid Hormone Economy in Critical Illness
Thyroid Hormone Economy in Critical Illness
Peripheral Metabolic Pathways
One of the initial alterations in thyroid hormone metabolism in acute illness is the acute inhibition of D1, resulting in the impairment of T4-to-T3 conversion in peripheral tissues.7 D1 is inhibited by a wide variety of factors, including acute illness (Box 166-1),2 resulting in the acute decrease in T3 production in critically ill patients. In contrast, inner ring deiodination by D3 may be increased by acute illness, resulting in increased levels of rT3.8 Additionally, because rT3 is subsequently deiodinated by D1, degradation of rT3 decreases, and levels of this inactive hormone rise in proportion to the fall in T3 levels. Finally, there is impaired transport of T4 to peripheral tissues such as the liver and kidney, where much of the circulating T3 is produced, further contributing to the decrease in production of T3.9
Thyrotropin Regulation
Serum TSH levels are usually normal early in acute illness.10 Decreased TRH secretion due to inhibitory signals from higher cortical centers, impaired TRH metabolism,11 the alteration of pulsatile TSH,12 and the decrease or absence of a nocturnal TSH surge12,13 may all further lower TSH levels. Serum levels of leptin, the ob gene product that has been shown to vary directly with thyroid hormone levels,14 also falls as illness progresses15 and hypothalamic TRH secretion falls, which in turn leads to lowered TSH levels.16
The decrease of hypothalamic TRH gene expression in animal models is, however, not associated with increased serum T4 and T3 levels.17 Finally, certain thyroid hormone metabolites that are increased during acute nonthyroidal illness may play a role in the inhibition of TSH and TRH secretion.18
Common medications used in the treatment of the critically ill patient may also have inhibitory effects on serum TSH levels (Box 166-2). Van den Berghe et al.19 reported that intravenous (IV) administration of dopamine for as short a time as 15 to 21 hours can acutely decrease TSH levels, and its withdrawal results in a 10-fold increase in serum TSH levels. In one study, children who received dopamine infusions during a pediatric ICU admission for meningococcal sepsis had lower TSH levels than those who did not.20,21 Increased levels of glucocorticoids, whether from endogenous or exogenous sources, also have direct inhibitory effects on TSH secretion.
Serum Binding Proteins
The affinity of thyroid hormones binding to transport proteins and the concentrations of serum binding proteins are altered with acute illness (Table 166-1). Serum levels of transthyretin and albumin decrease, especially during prolonged illness, malnutrition, and in high catabolic states. TBG levels may be increased, as seen with liver dysfunction and human immunodeficiency virus (HIV) infection, or decreased, as seen with severe or prolonged illness.5 TBG may also be rapidly degraded by protease cleavage during cardiac bypass, thereby partially explaining the rapid fall of serum T3 levels in patients undergoing cardiac surgery.22
TABLE 166-1 Factors That Alter Binding of T4 to Thyroid-Binding Globulin
| Increase Binding | Decrease Binding | |
|---|---|---|
| Drugs | Estrogens | Glucocorticoids |
| Methadone | Androgens | |
| Clofibrate | L-Asparaginase | |
| 5-Fluorouracil | Salicylates | |
| Heroin | Mefenamic acid | |
| Tamoxifen | Antiseizure medications (phenytoin, Tegretol) | |
| Raloxifene | Furosemide | |
| Heparin | ||
| Anabolic steroids | ||
| Systemic Factors | Liver disease | Inherited |
| Porphyria | Acute illness | |
| HIV infection | Nonesterified free fatty acids (NEFAs) | |
| Inherited |
An acquired binding defect of T4 to TBG is commonly seen in patients with critical illness. This is thought to result from the release of some as yet unidentified factor from injured tissues that has the characteristics of unsaturated nonesterified fatty acids (NEFA),23 which also inhibit T4-to-T3 conversion.24 In systemically ill patients, NEFA levels rise in parallel with the severity of the illness,25 and drugs such as heparin stimulate the generation of NEFA.26 Many drugs including high-dose furosemide, antiseizure medications, and salicylates also alter binding of T4 to TBG. The alterations in serum binding proteins in critical illness make estimating free hormone concentrations difficult (see later).
 Evaluation of Thyroid Function in the Critically Ill Patient
Evaluation of Thyroid Function in the Critically Ill Patient
Diagnostic Tests
Thyrotropin Assays
Abnormal thyroid function tests have been reported in 20% to 40% of acutely ill patients, more than 80% of whom have no intrinsic thyroid dysfunction after resolution of the illness.27–29 In a study of 1580 hospitalized patients, only 24% of patients with suppressed TSH values (TSH < assay limit of detection) and 50% of patients with TSH values over 20 mU/L were found to have thyroid disease.27,28 More importantly, none of the patients with subnormal but detectable TSH values and only 14% of patients with elevated TSH values less than 20 mU/L were subsequently diagnosed with intrinsic thyroid dysfunction. The development of sensitive third-generation TSH assays have led to small improvements in discerning between overt hyperthyroidism and nonthyroidal illness.27 Overall, however, while a normal TSH level has a high predictive value of normal thyroid function, an abnormal TSH value alone is not helpful in evaluating thyroid function in the critically ill patient.
Serum T4 and T3 Concentrations
Measurement of free thyroid hormone concentrations in the patient with nonthyroidal illness is fraught with difficulty.30 The gold standard for determination of free hormone levels is equilibrium dialysis. However, this technique is labor intensive and time consuming and thus is rarely used. The most commonly available laboratory tests of thyroid hormone concentrations, the free T4 index, free T4, and free T3, are measured by analog methods which represent estimates of the free hormone concentration and are therefore subject to inaccuracies.31,32
The free T4 index is determined by multiplying the total T4 concentration by the T3 or T4 resin uptake, which is an inverse estimate of serum TBG concentrations.32 Recent developments have allowed the measurement of free T4 levels by the analog method, a less expensive alternative to the free T4 index,33 but the two tests are likely comparably accurate.34 In a healthy population, there is a close correlation between the free T4 index and free T4 levels. In the critically ill patient, this association is no longer seen, mainly because of difficulties in estimating TBG binding with resin uptake tests. In spite of this, the sensitivity of the free T4 index in a large study of hospitalized patients was 92.3%, compared to 90.7% for the sensitive TSH test.27
Serum T3 concentrations are affected to the greatest degree by alterations in thyroid hormone economy resulting from acute illness. Therefore, there is no indication for routine measurement of serum T3 levels in the initial evaluation of thyroid function in the critically ill patient. This test should only be obtained if thyrotoxicosis is clinically suspected in the presence of a suppressed sensitive TSH and elevated (or high normal) free T4 index or free T4 values. The total T3 assay is preferable to the free T3 (analog) assay, owing to the variability between laboratories with the latter test.32
Although some investigators have reported that serum rT3 levels are a significant prognostic indicator of mortality in the ICU,35 rT3 levels are generally unreliable and should not be used to distinguish between intrinsic thyroid dysfunction and nonthyroidal illness.36
Serum Thyroid Autoantibodies
Autoantibodies to thyroglobulin and thyroid peroxidase (TPO), two intrinsic thyroid proteins, are commonly ordered tests.32 Significant titers of either or both of these antibodies indicate the presence of autoimmune thyroid disease, but the presence of thyroid autoantibodies alone does not necessary indicate thyroid dysfunction, as they are present in approximately 12% to 26% of the general population.37 Thyroid autoantibodies do, however, add to the sensitivity of abnormal TSH and FTI values in diagnosing known intrinsic thyroid disease.27,28
 Sick Euthyroid Syndrome
Sick Euthyroid Syndrome
As discussed earlier, critical illness causes multiple nonspecific alterations in thyroid hormone concentrations in patients without intrinsic thyroid dysfunction that relate to the severity of the illness.18,38,39 One author has postulated that sick euthyroid syndrome may be a compensatory mechanism in response to the oxidative stress of acute illness.40 Whatever the underlying cause, these alterations in thyroid hormone parameters represent a continuum of changes that depends on the severity of the illness and can be categorized into several distinct stages (Figure 166-2).18 The wide spectrum of changes observed often results from the differing points in the course of the illness when the thyroid function tests were obtained. Importantly, these changes are rarely isolated and often associated with alterations of other endocrine systems, such as decreases in serum gonadotropin and sex hormone concentrations41 and increases in serum ACTH and cortisol levels.42 Thus, the sick euthyroid syndrome should not be viewed as an isolated pathologic event but as part of a coordinated systemic reaction to illness involving both the immune and endocrine systems.
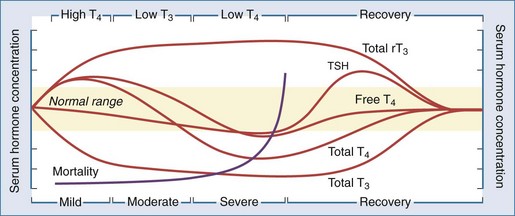
Figure 166-2 Alterations in thyroid hormone concentrations with critical illness.
(From Farwell AF. Sick euthyroid syndrome in the intensive care unit. In: Irwin RS, Rippe JM, editors. Intensive care medicine. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2003.)
Low T3 State
Common to all of the abnormalities in thyroid hormone concentrations seen in critically ill patients is a substantial depression of serum T3 levels, which can occur as early as 24 hours after the onset of illness. Over half of patients admitted to the medical service will demonstrate depressed serum T3 concentrations.27,28 Development of the low T3 state arises from impairment of peripheral T4-to-T3 conversion through inhibition of type 1 deiodinase (discussed earlier). This results in marked reduction of T3 production and rT3 degradation,43 thereby leading to reciprocal changes in serum T3 and serum rT3 concentrations. Low T3 levels are also found in peripheral tissues.35 Thyroid hormone receptor expression is also decreased in acute nonthyroidal illness,44 possibly in response to the decrease in tissue T3 levels.
High T4 State
Serum T4 levels may be elevated early in acute illness due to either the acute inhibition of type 1 deiodinase or increased TBG levels. This is seen most often in the elderly and in patients with psychiatric disorders. As the duration of illness increases, non-deiodinative pathways of T4 degradation increase serum T4 levels to the normal range.28
Low T4 State
As the severity and duration of the illness increases, serum total T4 levels decrease into the subnormal range. Contributors to this decrease in serum T4 levels are (1) a decrease in the binding of T4 to serum carrier proteins, (2) a decrease in serum TSH levels, leading to decreased thyroidal production of T4, and (3) an increase in non-deiodinative pathways of T4 metabolism. The decline in serum T4 levels correlates with prognosis in the ICU, with mortality increasing as serum T4 levels drop below 4 µg/dL and approaching 80% in patients with serum T4 levels below 2 µg/dL.45–47 Despite marked decreases in serum total T4 and T3 levels in the critically ill patient, free hormone levels have been reported to be normal or even elevated,30,31 providing a possible explanation for why most patients appear eumetabolic despite thyroid hormone levels in the hypothyroid range. Thus, the low T4 state is unlikely to be a result of a hormone-deficient state and is probably more of a marker of multisystem failure in these critically ill patients.
Recovery State
As acute illness resolves, so do the alterations in thyroid hormone concentrations. This stage may be prolonged and is characterized by modest increases in serum TSH levels.48 Full recovery with restoration of thyroid hormone levels to the normal range may require several weeks49 or months after hospital discharge.27 One study reported that 35 of 40 patients with nonthyroidal illness after coronary artery bypass grafting were able to regain normal thyroid function 6 months after surgery.50
Treatment Of The Sick Euthyroid Syndrome
The question of whether the sick euthyroid syndrome in critically ill patients represents pathologic alterations in thyroid function that negatively impact these patients or simply reflects the multisystem failure (i.e., respiratory, cardiac, renal, hepatic failure) that occurs in critically ill patients is still debatable.51–54 What is not debatable is that thyroid hormone replacement therapy has not been shown to be of benefit in the vast majority of these patients in the published studies to date (Box 166-3).54 Evidence does suggest a beneficial effect of liothyronine (L-T3) on increasing organs available for harvest from brain-dead organ donors. While L-T3 appears to slightly improve hemodynamic and neurohumoral parameters in patients with congestive heart failure, these benefits may represent a pharmacologic effect of T3 rather than a physiologic replacement hormonal effect. Further, the studies involving patients with congestive heart failure are more remarkable for a lack of deleterious effect of L-T3 treatment then for any sustained clinical benefit. However, future studies do appear to be warranted in this patient population. At the present time, in the absence of any clinical evidence of hypothyroidism, there does not appear to be any compelling evidence for the use of thyroid hormone therapy in any patient with decreased thyroid hormone parameters due to the sick euthyroid syndrome.