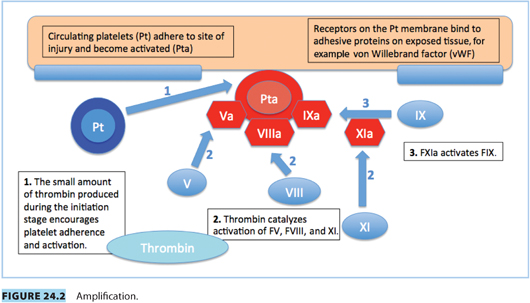
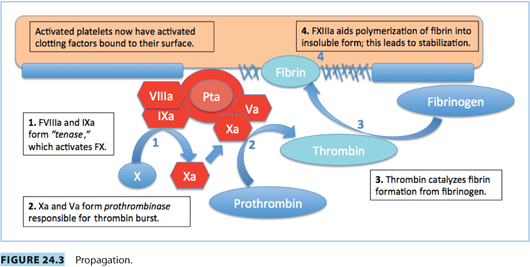
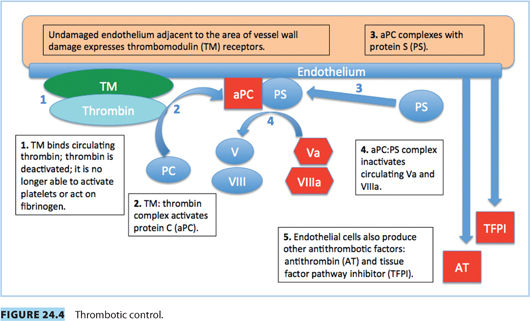
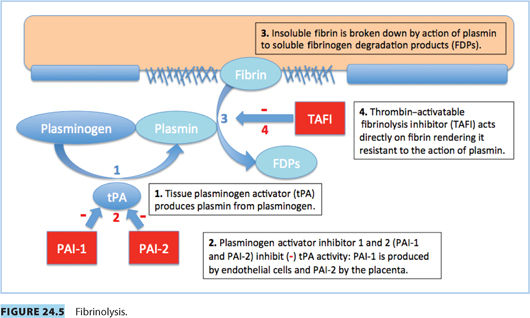
Vascular spasm, mediated by vasoconstrictor substances released from platelets, reduces blood flow and occurs in the first stages of hemostasis. Damaged endothelium attracts platelets, which adhere to it, forming a platelet plug. A clot forms as the platelet plug is stabilized with fibrin.
Hemostasis occurs in several phases.
II. Physiologic changes of coagulation in pregnancy
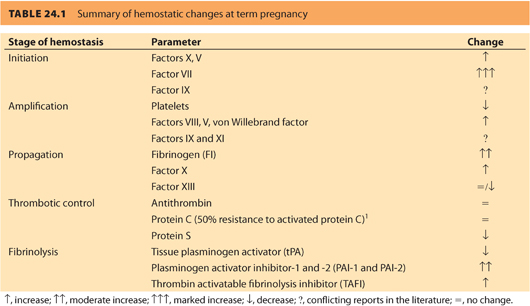
In general, there is an increase in most coagulation factors and a decrease in native anticoagulants and fibrinolytics. As a result, normal pregnancy is associated with a sixfold increase in VTE. Incidence is highest in the third trimester and postpartum. Because of the physiologic changes that occur in coagulation with pregnancy, reference ranges are different from the normal population. Changes at term are summarized in Table 24.1.
A. Platelets2
1. Platelet count decreases throughout pregnancy, is lowest at term, and normalizes 4 to 6 weeks postpartum
2. Decrease is due to the destruction by the uteroplacental unit and dilution by expanded plasma volume
3. Production and function are normal, lifespan is decreased, and mean volume increased.
B. Coagulation factors3
1. FVIII, X, XII, and von Willebrand factor (vWF) increase.
2. FVII may increase by up to tenfold.
3. Fibrinogen can double.
4. FXIII increases in the first trimester, but falls to half of prepregnancy levels by term.
5. Reports on FIX and FXI levels are conflicting.
C. Thrombotic control3
1. Protein C (PC) levels remain stable or slightly increased.
2. Protein S (PS) levels decrease.
3. Antithrombin levels are unchanged.
D. Fibrinolysis3
1. Tissue plasminogen activity (tPA) is reduced.
2. The decrease is the result of increased plasminogen activator inhibitor-1 and -2 (PAI-1 and PAI-2); PAI-2 is primarily produced by placenta
3. Thrombin-activatable fibrinolysis inhibitor (TAFI) is increased by term.
III. Measurements of coagulation in pregnancy
A. Routine tests
When combined with a clinical history, routine tests act as a screening tool to exclude coagulopathies. As highlighted by the cell-based model, these routine tests may not represent what is happening in vivo.
1. Platelet count as part of a complete blood count (CBC)
2. Prothrombin time (PT) or international normalized ratio (INR) measures the extrinsic and common pathways (see Fig. 24.6).
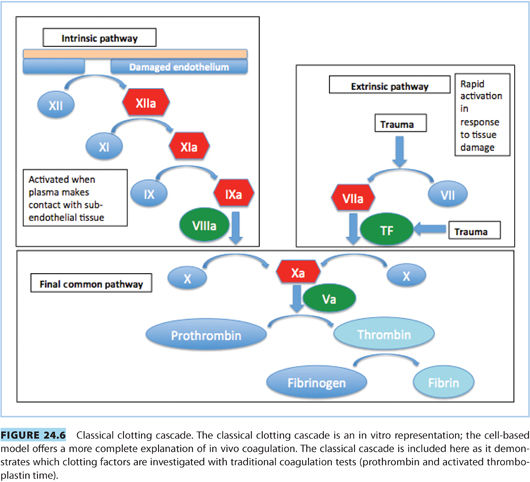
3. Activated partial thromboplastin time (aPTT) measures the intrinsic and common pathways (see Fig. 24.6).
4. Fibrinogen. There has been recent interest in the fibrinogen level taken at first diagnosis of postpartum hemorrhage (PPH) as a predictive marker of the severity of PPH. If fibrinogen is <2 g per L, the adjusted odds ratio for severe PPH is 12.4
B. Bleeding time
Although an in vivo measure of hemostasis, its clinical use is limited. It is invasive, and the results are operator dependent. It is nonspecific and nonsensitive at predicting perioperative bleeding.5
C. Near patient testing
1. Proposed roles of near patient testing:
a. Replace formal CBC for emergent surgery where neuraxial anesthesia is considered.
b. Assess platelet function in parturients with thrombocytopenia.
c. Screen for suspected platelet dysfunction, for example, those with a history of easy bruising/bleeding.
d. Assess anticoagulant status, for example, after stopping heparin therapy.
e. Guide blood product replacement in obstetric hemorrhage.
2. Currently, there is no evidence to support the ability of near patient testing to predict risk of clinical bleeding, for example, the risk of epidural hematoma with neuraxial anesthesia.
a. Thromboelastography6–8 or thromboelastometry9–11
(1) Dynamic viscoelastic tests: provide a visual representation of hemostasis: clot initiation, formation, stability, breakdown
(2) Global assay of whole blood
(3) Platelet function is represented by maximum amplitude (MA); MA is also influenced by fibrinogen levels.
(4) MA is increased in normal pregnancy, representing the hypercoagulable state.8
(5) Disadvantage: the tests do not detect effect of antiplatelet therapy, for example, aspirin
b. Platelet function analyzer (PFA 100)6,7,12
(1) Represents primary hemostasis as closure time (CT): the time taken for platelet plug to form in an aperture when whole blood is placed on a collagen sheet
(2) One study supporting PFA as a test for platelet quality and quantity suggested CT increases in healthy parturients with a platelet count <80 × 109. l−1 and was more sensitive than thromboelastography (TEG) at identifying platelet dysfunction associated with preeclampsia in those with a normal platelet count.7
IV. Thrombophilia
Thrombophilia is associated with the following thromboembolic complications:13
• Arterial and VTEs
• Recurrent fetal loss
• Intrauterine growth restriction
• Preeclampsia with severe features
• Placental abruption
A. Risk factors for thromboembolic disease in pregnancy
1. Virchow’s triad: the causes of thrombosis
a. Hypercoagulability
(1) Hemostatic changes of pregnancy (Table 24.1)
(2) Inherited thrombophilia (see Section IV.B.)
(3) Acquired thrombophilia
(a) Antiphospholipid syndrome (APS)
(b) Nephrotic syndrome (antithrombin loss)
b. Stasis
(1) Venous distension
(2) Mechanical obstruction to venous return from lower limbs by the gravid uterus
(3) Bed rest
c. Endothelial injury
(1) Cesarean delivery
(2) Traumatic vaginal delivery
2. Additional risk factors
a. Obesity
b. Age >35 years
c. Infection
3. Although risk factors increase the incidence of VTE, events frequently occur in parturients without risk factors.14
CLINICAL PEARLPostpartum anticoagulation
Because VTE is a major cause of maternal morbidity and mortality, particularly after operative delivery, a way to improve patient outcome is to assess the risk of VTE in all patients undergoing cesarean delivery and anticoagulate those at high risk. Specific protocols should prompt physicians to assess the postpartum VTE risk for each parturient and include dosing and timing schedules for low molecular weight heparin (LMWH) relative to time of neuraxial procedures (including neuraxial catheter removal) and surgery. One approach to ensure that anticoagulation is considered after operative delivery is to include VTE risk and anticoagulation as part of the Surgical Safety Checklist.
B. Inherited thrombophilias
Inherited thrombophilias are responsible for 30% of maternal VTE; half of these are a result of either Factor V (FV) Leiden or prothrombin gene abnormalities.15,16 Genes associated with increased VTE risk are carried by 15% of the Caucasian population.17
1. Factor V Leiden
a. Most prevalent inherited thrombophilia13
b. Prevalence highest in European Caucasian populations
c. Mutation in FV gene, rendering FV resistant to inactivation by activated PC (Fig. 24.4). This leads to slower breakdown of FV, increased thrombin production, and a prothrombotic state.
d. Heterozygous state common: rarely leads to VTE unless combined with another risk factor, such as pregnancy
e. Homozygous state increases VTE risk by up to 80 times.17
2. Prothrombin gene mutation
a. Point mutation (G20210A)
b. Increases the circulation of functionally normal prothrombin
c. Most VTEs occur in those patients with additional risk factors
d. Commonly inherited with FV Leiden. With both mutations: 100-fold increase in VTE.16
3. Antithrombin deficiency
a. Previously known as antithrombin (AT) III deficiency
b. Most severe of the inherited thrombophilias
c. AT is a natural anticoagulant that binds and deactivates thrombin: AT decreases the production and half-life of thrombin. It also binds and deactivates Factors IXa, Xa, XIa, and XIIa (Fig. 24.4).
d. Parturients with normal AT activity, but decreased quantity, have a 400 times increased risk of VTE.15
e. There are greater than 250 mutations that either reduce quantity or quality of AT production
f. Homozygous individuals are rare: most die in utero.
g. AT deficiency can be acquired, for example, liver disease, malnutrition, or consumptive coagulopathy.
4. Protein C deficiency
a. Natural anticoagulant, degrades FVa and FVIIIa
b. Strong inhibitor of coagulation, activated by the thrombin/thrombomodulin complex in the presence of protein S (a cofactor)18 (Fig. 24.4)
c. PC deficiency results from more than 160 distinct mutations, with variable quality and quantity of PC produced.13
5. Protein S deficiency
a. Is a cofactor for activated PC
b. Also directly inactivates FVa and FXa
c. Normal pregnancy increases protein binding of PS, reducing the availability of unbound active PS.
(1) Individuals homozygous for either PC or PS deficiency present with neonatal purpura fulminans and require lifelong anticoagulation.13
C. Management of parturients with suspected thrombophilia
1. Screening for thrombophilia
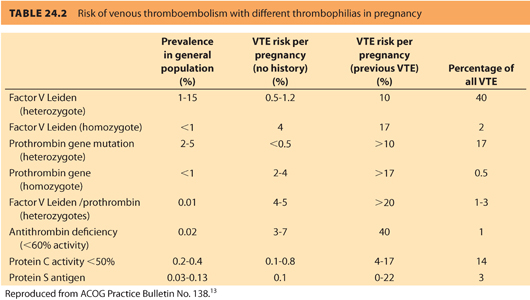
a. Screening results guide individual risk assessment and decisions about anticoagulation. Testing for all genetic mutations listed in Table 24.2 should take place prior to pregnancy in parturients with:
(1) Previous history of a VTE
(2) First-degree relative with a history of thrombophilia
b. Pregnancy, recent thrombosis, and anticoagulation treatment can affect test results.13
2. Treatment
a. Extent of anticoagulation therapy based on an individual risk assessment depending on:
(1) Prior personal and family history of VTE
(2) Presence of genetic mutation
(3) Additional risk factors, for example, immobility, obesity, cesarean delivery13
b. VTE risk is greater in the first 6 weeks postpartum than during pregnancy: Anticoagulation needs to be continued, or increased, postpartum.
c. Inherited thrombophilias are divided into low and high risk; this guides anticoagulation management (Table 24.3).13
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







