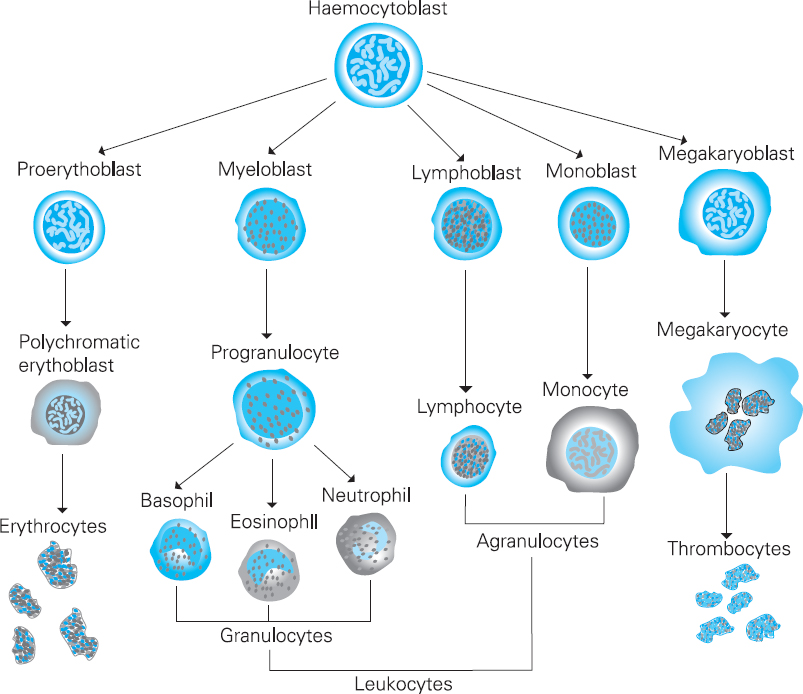
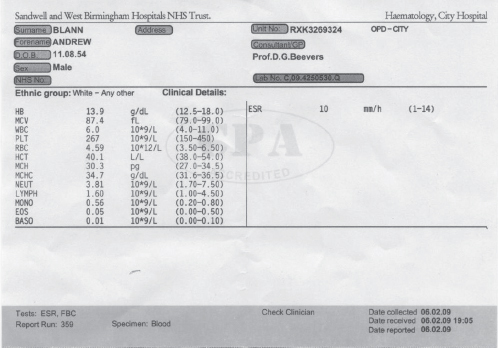
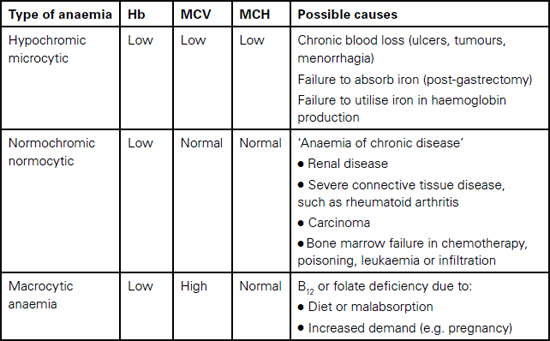
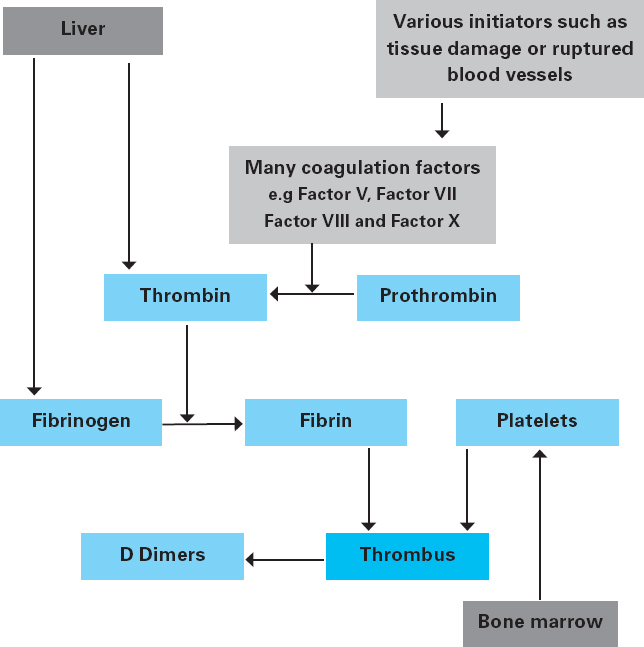
Andrew Blann This chapter will outline: • the basis of the main haematological investigations • what these blood tests can tell us about the patient • red blood cells and white blood cells in surgery • the role of coagulation and anti-coagulation in surgery. Haematology involves the study of the form and function of the following blood components: red blood cells, white blood cells, platelets and coagulation proteins, the latter two components being important to form a clot (or thrombus). The haematology laboratory commonly provides support for preoperative assessment in the form of haematology investigations of which the most frequently requested is the full blood count and the coagulation screen. These tests will allow the preoperative assessor a view of the ability of the patient’s blood to deliver oxygen to the tissues, and also an opportunity to pick up any abnormality that may affect the patient during the perioperative period, especially with respect to the patient’s coagulation status. The results of these investigations may require the practitioner to instigate changes in the patient’s on-going care in an effort to avoid the potential delay or cancellation of this surgery. Common issues identified relate to either anaemia or a coagulopathy but may identify previously unidentified disease states such as leukaemia or aplastic anaemia. The haematology laboratory will therefore offer additional support to the practitioner in providing the expertise to advise and to agree to these changes in consultation with other members of the multi-disciplinary team such as the surgeon and the anaesthetist as well as providing information for the patient. These changes should be instigated well in advance of planned surgery but may need expediting if required in the emergency setting, such as reversal of anti-coagulation prior to emergency surgery. There are three types of cells found in blood – red blood cells (erythrocytes), white blood cells (leucocytes) and platelets (thrombocytes). These are produced in the bone marrow from differentiation of stem cells in response to stimuli such as hormones, whose production can be increased in response to pathological processes such as infection and sepsis. An outline diagram (Figure 10.1) shows the differentiation of the stem cells into the various categories of blood cells. Figure 10.1 The development of blood cell types from the undifferentiated stem cells in the bone marrow. The white blood cells (leucocytes) can be divided into granulocytes and agranulocytes, the former consisting of basophils, eosinophils and neutrophils and the latter consisting of lymphocytes and monocytes. Further information about the differentiation of the blood cell types and the pathological diseases such as leukaemia can be found in specific haematology textbooks. The production of these blood cells can be affected by pathological processes and this may lead to failure to produce sufficient red blood cells, which will lead to anaemia, failure to produce sufficient white blood cells (leukopenia), which will lead to infections, whilst insufficient platelets (thrombocytopenia) will lead to bruising and bleeding. Each of these low levels may be present by themselves, though low levels of all three types occur simultaneously – this is known as pancytopenia. This is often seen in patients undergoing chemotherapy and radiotherapy associated with cancer, as the bone marrow is very sensitive to these toxic drugs. Routine monitoring is required to evaluate the impact of these drugs on the bone marrow of such patients. The most common serious bone marrow disease is leukaemia, which is caused by a failure to correctly regulate the number of white blood cells being produced. Not only are there increased numbers of white blood cells generated, but also those that are produced are immature and cannot fulfil their function of defending us from infections. As the tumour of immature white blood cells grows inside the bone marrow, the production of the two other types of blood cells, red blood cells and platelets, is also affected, and levels fall, leading to anaemia and thrombocytopenia. The two commonest requested haematological investigations from the preoperative assessment clinic are the full blood count (FBC) and the coagulation screen. Historically an allied test – the ESR or erythrocyte sedimentation rate – was requested as a measure of the physical property of the blood. Its use has reduced significantly over the last two decades as it shares common ground with the ‘gold standard’ serum marker of inflammation – C reactive protein (CRP). It is now rarely requested within the preoperative assessment environment. Full blood count and coagulation screen require blood to be collected into specific blood collection bottles, each requiring a specific anticoagulant prior to processing. FBC requires the anticoagulant ethylene diamine tetra-acetic acid (EDTA), while the coagulation screen requires the anticoagulant sodium citrate. Practitioners must check with the laboratory of their specific healthcare organisation as to the particular specifications of their organisation’s blood collection tubes as this may vary with geography and supplier. Collection into the incorrect tubes will require duplication of the investigation, much to the annoyance of the patient, and may delay the eventual surgical procedure. The FBC provides numerous results: those of red blood cells, of white blood cells, and of the number of platelets. An example of the results of a full blood count is displayed in Figure 10.2. Figure 10.2 Full blood count result of a middle-aged healthy male patient, showing information relating to the three cellular components, red blood cells, white blood cells and platelets Red blood cells The important red blood cell measurements are haemoglobin (Hb), the red blood cell count (RBC in Figure 10.2) and haematocrit (Hct). There are also three other red cell indices: mean cell volume (MCV), mean cell haemoglobin (MCH) and mean cell haemoglobin concentration (MCHC) – the MCV being the most useful. Haemoglobin is undoubtedly the index most frequently referred to in clinical haematology. It is a protein designed to carry oxygen from the lungs to the tissues, where the oxygen is given up to participate in respiration. The normal range varies between the sexes. Lower levels in menstruating women seem obvious, but in post-menopausal women levels are still lower than age-matched men as the latter produce testosterone to stimulate red cell production. Haemoglobin is carried in the blood in red blood cells, the number of which is provided as the red blood cell count (RBC). Red cells are unusual as they lack a nucleus, which gives additional flexibility, allowing the cells to penetrate the smallest capillaries. They are the most abundant cell in the blood, are often called erythrocytes, and numbers can also vary between the sexes, being lower in women. The haematocrit (Hct) expresses that proportion, as a decimal or as a percentage, of whole blood that is taken up by all the blood cells. Since there are approximately a thousand more red blood cells than both white blood cells and (tiny) platelets, the red cells make up the major proportion of the haematocrit. Consequently, at the practical level, it provides an idea of the proportion of red blood cells that makes up the whole blood pool. The other red blood cell indices are the mean cell volume (MCV), which is the size of the average (mean) red blood cell. Note the stress is on ‘average’, as each index is the mean of millions of individual cells. The mean cell haemoglobin (MCH), as the name implies, simply reports the average amount (mass) of haemoglobin in the average cell. It does not take into account the size of the cell. Mean cell haemoglobin concentration (MCHC) is the average concentration of haemoglobin inside the average size cell. These indices are useful in diagnosing causes of anaemia, such as a microcytic anaemia which occurs with iron deficiency or a macrocytic anaemia with folate deficiency. White blood cells The white blood cell indices reported include the white cell count (WCC) and the differential. The latter is actually five different results – it tells us of the different proportions of the various types of white cells. These are (in order of frequency) neutrophils, lymphocytes, monocytes, eosinophils and basophils (see Figure 10.2). All these cells perform different tasks in defending us from attack by micro-organisms, and additionally take part in processes such as hypersensitivity reactions, e.g. hay fever. There may also be some unusual cells called blasts or atypical cells, and in high numbers these are likely to have considerable adverse consequences. Platelets These tiny bodies are fragments of a much larger cell found only in the bone marrow (the megakaryocyte). With no nucleus, they consist only of cytoplasm, but this extremely potent cytoplasm is packed full of chemicals. Platelets form a clot, or thrombus, when aggregated together with the help of the blood protein fibrin, and so minimise haemorrhage. This generally consists of a plasma protein (fibrinogen), two clotting times (PT and APTT), and two ratios (INR and PTT ratio). Fibrinogen is one of the more important blood proteins involved in clotting and is made in the liver. It is converted into fibrin by an enzyme, thrombin (itself derived from prothrombin, and also a product of the liver), and is crucial in clot formation. Prothrombin time (PT) and partial thromboplastin time (PTT) are measures of the ability of blood plasma to form an artificial clot in the laboratory. Some labs place an ‘A’ for ‘activated’ in front of the PTT, thus APTT. The difference between PT and PTT is that they measure different parts of the clotting pathway, can investigate different bleeding disorders, and can also be used to monitor the effects of different drugs that interfere with different parts of the coagulation system. The PT is commonly used to assess the efficacy of the use of the oral anticoagulant warfarin which works on (some would say poisons!) the liver to prolong the PT and so make a clot less likely to happen. We use a ratio between the normal PT (i.e. when not on warfarin) and the PT whilst the patient is on warfarin to generate the international normalised ratio (INR). Similarly, the effectiveness of the injectable anticoagulant unfractionated heparin is monitored by its effect on prolonging the PTT, which also delays clot formation. Hence someone on this heparin will have a prolonged PTT, and we use a ratio between these two versions of the PTT to ensure the correct dose of the drug: hence the PTT ratio. However, unfractioned heparin is slowly giving way to an improved preparation called low molecular weight heparin (LMWH), which does not generally need laboratory monitoring. The importance of these times, the INR, and the PTT ratio will be explained in a later section. The question of what preoperative tests are required in the preoperative assessment clinic has been raised for several years. The past practice of requesting all investigations for all patients, regardless of their medical fitness or the surgical procedure that is being performed, is legendary in the teachings among many junior medical staff who historically performed the patient’s ‘clerking in’ on admission or in an outpatient clinic. Thankfully this approach has been challenged both from a cost perspective and from an evidence-based point of view. The National Institute for Clinical Excellence released Clinical Guidance 3 in June 2003 titled ‘Preoperative tests: The use of routine preoperative tests for elective surgery’.1 The guidelines were based upon the best available evidence, which is very limited, being only level IV evidence (that is, expert opinion derived from a consensus development process and the clinical experience of the Guideline Development Group). The NICE guidelines, however, can be used as a framework, firstly for developing local guidance and secondly to improve the evidence base in forthcoming research. In evaluating the indices of blood tests requested, the provision of a reference range (sometimes called the normal range, or the target range) helps the practitioner in deciding if further investigations are required. There is a hope that all results will be within the reference ranges, though there are three important points to remember: • A result in the reference range does not necessarily make the patient well, but it certainly does reduce your level of anxiety! • And in reverse, just because a result is outside the reference range does not necessarily mean the patient is at death’s door. However, the further the result is away from the reference range, then the more concerned you must be. • References ranges are not carved in granite: they change with time and between healthcare organisations. Broadly speaking, we need to consider results which are above or below the reference range. A full blood count can indicate two possible major abnormalities with red blood cells: 1. Not enough red blood cells – anaemia 2. Too many red blood cells – erythrocytosis or polycythaemia. Polycythaemia is rare, though uncontrolled it has been implicated as the only abnormality of circulating blood cells that has a positive impact on perioperative morbidity. The condition may cause a possible fatal stress on the heart and cardiovascular system and may precipitate a stroke. The use of elective venesection is used to control the disease, though thorough investigation of the underlying conditin will be required as a matter of urgency. Table 10.1 Anaemia and its causes Anaemia is a far more common diagnosis that is demonstrated with the full blood count. Anaemia may be defined as the clinical (symptomatic) consequence of the failure of the blood to deliver enough oxygen to the tissues so they can adequately perform their physiological function. In contrast, other authorities will define anaemia as a level of haemoglobin below a certain level. However, a haemoglobin level of, say, 11.5g/dL may well be perfectly adequate for an elderly woman with few physiological requirements and a relatively quiet life, while the same haemoglobin level in a younger person with a very active lifestyle, perhaps including sports, will be inadequate. Thus the medical state of the individual as a whole person should be considered, not merely an arbitrary number at which one acts. An alternative view of anaemia may be the level at which concern arises, and at which further investigations are considered. Certainly, anaemia should not be seen merely as that level that automatically requires a blood transfusion, a therapy that many consider should be reserved only for life-saving situations. The cause of anaemia should be elucidated prior to elective surgery as much as possible. The use of all the indices reported with a full blood count will assist with this diagnosis by determining the size and haemoglobin content of the circulating red blood cells. Table 10.1 (page 193) outlines common findings. White blood cells, or leukocytes, are collectively responsible for defending us from attack by micro-organisms such as viruses, bacteria and parasites. Accordingly, raised levels of these cells (which is described as leukocytosis) can be expected in the face of infections with these microbes. However, increased numbers may also be present in a number of conditions such as rheumatoid arthritis and cancer, and also after surgery and in leukaemia. It follows, in reverse, that low levels of white blood cells (leucopenia) may be a sign that the patient could be susceptible to infections. However, the causes of unexplained low white blood cells are very rare, and of these the most common is aplastic anaemia, and if present, there is also a low red blood cell count. The most common reason for leucopenia is the effect of cytotoxic drugs on the bone marrow, a therapy only undertaken in severe disease such as cancer. In this case, the patient is most likely to be aware of this problem. The most common white blood cells are neutrophils and lymphocytes. Low levels of neutrophils and lymphocytes are, respectively, described as neutropenia and lymphopenia. Similarly, an increased number of neutrophils is called neutrophilia, or perhaps a neutrophil leukocytosis. High numbers of lymphocytes are referred to as lymphocytosis. Neutrophils and lymphocytes have different functions. The fundamental purpose of the neutrophils is to protect the body from bacterial infections, such as may be caused by staphylococcal and streptococcal organisms. One mechanism by which neutrophils perform this function is to engulf the bacteria, and then kill and digest them with the help of intracellular enzymes and chemicals (phagocytosis). Another of the white blood cells, the monocyte, is also able to digest bacteria. Hence both neutrophils and monocytes may be described as phagocytes. Consequently, high numbers of neutrophils are often associated with bacterial infections, but may also be found in autoimmune diseases such as rheumatoid arthritis. This is because in arthritis the immune and inflammatory systems attack the body’s own tissues (such as the skin and joints) instead of bacteria. Increased levels of neutrophils in the absence of an infection, such as after surgery or severe exercise, are referred to as the ‘acute phase response’. The acute phase response is a collection of physiological actions that are generated by a shock to the body, often microbiological or traumatic, including iatrogenic trauma of surgery. The body is programmed to perceive this shock as a potential threat, and so initiates steps to protect itself. A typical shock is that of surgery; another is a severe infection. The actual changes of the acute phase response include a rise in blood pressure, in white blood cells (to defend against presumed microbial attack), and increase in platelet count and coagulation proteins (to defend against potential haemorrhage). An important feature of the acute phase response is that raised levels are to be expected to fall back to normal as the cause of the stress subsides. There are two major types of lymphocytes: B lymphocytes make immunoglobulins (antibodies – proteins designed to recognise, attack, and help destroy invading pathogens – Figure 10.1, page 188) whilst T lymphocytes cooperate in antibody production and also attack cells that are infected with viruses. Whilst many lymphocytes are present in the blood, they are also to be found in lymph nodes, the bone marrow, the liver and the spleen. These organs are collectively called ‘lymphoid tissue’. Mildly increased numbers of lymphocytes in the blood may be expected in conditions that also cause a raised neutrophil count. However, the highest levels often encountered in health are found during attack against viral infections, such as glandular fever. In such cases these ‘activated’ lymphocytes may be larger than usual, and sufficiently large to be described as blasts. However, some viruses actually kill lymphocytes, so that the number in the blood will fall, HIV being such a virus. Haemostasis is the technical name for the balance between an increased risk of clotting (thrombosis) and an increased risk of bleeding (haemorrhage). If this balance is disturbed then clinically important disease may result. Good haemostasis, as evidenced by ‘healthy’ clot formation, requires the correct activation and numbers of platelets with the correct assembly of coagulation proteins. An increased risk for thrombosis follows from increased numbers of platelets (called thrombocytosis) and high levels of the coagulation proteins such as fibrinogen. However, platelets and fibrinogen do not normally come together to form a clot; the process of thrombosis generally requires a trigger. A good example of such a stimulus is orthopaedic surgery, which is why patients undergoing these operations are at risk of thrombosis and so need protection with anticoagulants. Thrombocytosis is often present in infections and in autoimmune diseases such as rheumatoid arthritis. An increased risk of haemorrhage may result from low numbers of platelets (known as thrombocytopenia) and/or low levels of coagulation proteins. Drugs such as quinine and sulphonamides may cause a low platelet count, as well as poor production from the bone marrow, or excessive consumption. Low levels of coagulation proteins will be demonstrated by prolonged prothrombin and partial thromboplastin times. However, in the absence of established drug therapies such as warfarin and heparin, excess clotting times are hardly ever encountered in a routine setting. Perhaps the best-known such disease is haemophilia, caused by the lack of coagulation Factor VIII, but if present, it will have been present from childhood and very obvious to the patient and his family. The whole topic of coagulation is very important in surgery and will be developed in great detail in a later section. Figure 10.3 (page 196) shows how the coagulation system comes together to form a clot (thrombus). Figure 10.3 Simplified diagram of the coagulation system Several factors start the coagulation ‘cascade’, such as crush injuries, severe bacterial infections or severed blood vessels that expose collagen. Among the first coagulation factors to be activated are Factor V and Factor VII. Later, Factor X becomes activated and so is referred to as Factor Xa. The activity of this molecule is inhibited by low molecular weight heparin (LMWH), and Factor Xa is a major contributor to the complex of Factors and other molecules whose function is to convert prothrombin into thrombin. Once formed, thrombin acts on fibrinogen to turn it into fibrin. Fibrin forms a mesh that, with platelets and other blood cells, generates a thrombus (clot) to reduce blood flow. Table 10.2 briefly summarises the value of the full blood count and coagulation screening tests in preoperative assessment. Table 10.2
Chapter 10 The role of haematology in preoperative assessment
SUMMARY
INTRODUCTION
The origin of blood cells
Haematological investigations
Full blood count (FBC)
Coagulation screen
What tests and when?
Red blood cells
White blood cells
Platelets and coagulation proteins
Interpretation of major haematological tests in preoperative assessment
Red blood cells • Is the patient anaemic? • Can red blood cells provide enough oxygen to the tissues to allow them to perform their functions whilst the patient is being operated upon? • If borderline anaemic, can the patient withstand any loss of blood during surgery? • If so, should a number of units of packed red cells be prepared by the Blood Bank? |
White blood cells • If mildly or moderately raised: is the patient experiencing a current infection? If so, there is also likely to be a raised CRP. • If markedly raised: does the patient have a serious disease such as leukaemia or lymphoma? • If profoundly low: the patient may be at risk of an infection. It follows that you may well ask ‘why is the patient’s white blood cell count profoundly low?’ |
Platelets and coagulation proteins • If thrombocytopenic (platelet count < 100): the patient may be at risk of haemorrhage, and especially so if the platelet count is < 50. • A prolongation of PT and PTT is inevitably caused by a particular disease (such as haemophilia) or by anticoagulant drugs. |
Red blood cells and white blood cells in surgery
The haematology laboratory provides the practitioner with important information not merely about the patient’s general health, but also provides clues about how they may react to their surgery. As with all blood tests, the primary question in a screening setting would probably be ‘Is the patient fit for surgery?’
The red blood cell, haemoglobin, and anaemia
The major concern about red blood cells before, during and after surgery, is anaemia. Although high number of red cells can be dangerous, there are few patients with this problem. However, even the textbook definition of anaemia can vary, such as a haemoglobin result of less than 12g/dL, or less than 11g/dL, or even lower values, and perhaps varying with sex. Others define anaemia as the point at which the body may suffer, or perhaps when treatment is needed. Consequently, in the light of impending surgery, a high degree of flexibility is called for.
Before surgery
The function of the red blood cell and haemoglobin is to deliver oxygen to the tissues. However, surgery increases the demand by the body for oxygen. Thus a mild or moderate anaemia may not be a problem without surgery, but under stress a smaller number of red cells may not be able to provide the amount of oxygen required. This may have severe consequences, such as a heart attack. It may also be the case that anaemia is caused by a more dangerous and hidden disease, such as pure red cell aplasia, which means that the patient may not be able to withstand the effects of the surgery.
Nevertheless, if anaemia is present, it may be treatable in the short or moderate term. If so, then this is certainly worth considering, especially if the surgery can be delayed, as in elective orthopaedic cases. If this option is taken, the patient will need to be passed to the haematologists for treatment. The time taken for anaemia to resolve depends on the basis of the disease. For example, iron-deficient anaemia may respond to oral, depot or infused iron within a month or six weeks. However, the cause of the anaemia must be investigated, as it may be related to other serious disease such as myeloma, although most common diseases can be detected by more than one sign, symptom or laboratory result. There are many possible causes of anaemia, as indicated in Table 10.3. The practitioner will be aware that few of these conditions are associated with a single abnormal result and that a broad knowledge is required to successfully confirm a diagnosis.
Table 10.3 Potential causes of anaemia
Problems with the bone marrow • Bone marrow cancer (leukaemia, lymphoma, myeloma) • Secondary cancer (originating elsewhere, e.g. breast, prostate) • Suppression (perhaps by chemotherapy) |
Lack of micronutrients • Insufficient iron and vitamin B12 in the diet • Good diet, but malabsorption due to intestinal disease |
Disease in other organs • Liver disease – this organ stores iron and synthesises crucial proteins • Renal disease – if damaged, the kidney may fail to make the growth factor erythropoietin |
Destruction of mature red blood cells • Haemolytic anaemia • Infection, such as malaria • Haemoglobinopathy (sickle cell disease, thalassaemia) |
Physical loss of blood • Heavy menstrual periods • Via a cancer in the lower intestine (colon, rectum) |
If the need for the surgery is vital, perhaps acting on a life-threatening condition, then an alternative approach is to perform a short-lived but effective treatment – a blood transfusion. This is discussed in more detail in Chapter 11 of this book.
The effects of surgery
Clearly, some types of surgery can be more ‘bloody’ than others, especially those involving arteries. But there are several strategies to minimise blood loss, such as salvaging, followed by recycling. Despite this, surgical procedures seemingly without notable ‘on table’ blood loss are still associated with a fall in haemoglobin. In some cases of orthopaedic surgery, haemoglobin levels measured in the postoperative period have not reached pre-surgery levels by six weeks. Very accurate measures of blood loss indicate that the loss of a litre of blood should, in theory, lead to a fall in haemoglobin of 2.5g/dL. But in practice, this level of blood loss regularly leads to a fall of 3g/dL. This increased fall above and beyond the simple physical blood loss of surgery is well established but cannot be explained by unmeasurable blood loss (e.g. via the intestines). A healthy response to surgery sees the mobilisation of inflammatory cytokines as part of the acute phase response, and these may influence iron kinetics. For example, even simple surgery such as arthroscopy of the knee or correction of hallux valgus (with minimal blood loss) can cause a significant decrease in serum iron.
Recovery after surgery
This will be associated with a degree of reconstruction of cells and tissues and so there will be a high demand for oxygen. It is possible, therefore, that postoperative anaemia may impair recovery. However, the spleen is an effective reservoir of red blood cells, and the fact that the spleen shrinks after surgery suggests that some of these stored red cells have entered the circulation. As indicated in the previous paragraph, the red blood cell count and haemoglobin count may take months to recover.
However, red blood cell production can be actively promoted by the provision of the growth factor erythropoietin. Given subcutaneously in the perioperative period, this agent is effective in increasing levels of haemoglobin and in reducing the need for blood transfusions.
Is anaemia always bad?
It follows that the ‘normal’ response to surgery is to reduce the haemoglobin level. This is contrary to the clear presumption that anaemia is a disease that demands treatment. Certainly, there are numerous diseases (such as cancer, chronic heart failure and chronic renal failure) where low iron status is associated with a poor prognosis, but this may be coincidental. However, this is too simplistic since not all treatments (such as blood transfusion) are safe and beneficial. It has been argued that, in fact, some forms of anaemia are an adaptive response that may be beneficial. For example, it is established that high iron levels can promote the formation of noxious reactive oxygen species. Additionally, iron is an essential requirement of several species of pathogenic bacteria, so that minimising free iron may also restrict the growth of these organisms.
White blood cells and microbes
Leucocytes defend us from microbial attack. So whereas high levels may indicate an infection, low levels may predispose to an infection.
Before surgery
A low white blood cell count may be found in an assessment clinic. In the absence of cytotoxic chemotherapy (possibly anti-cancer, and which the surgeon and various healthcare professionals are likely to be aware of) a low white cell count is important and needs investigating. This is not merely because it may be linked with other and/or more severe diseases, but also because it may leave the patient open to attack by pathogenic microbes. Therefore, if surgery cannot be postponed, antibiotic (if neutrophils are low) or antiviral (if lymphocytes are low) prophylaxis may be necessary. However, no drug is without a side effect, and, ironically, one of the most common such side effects is suppression of the bone marrow. A compromised white cell response after surgery may lead to life-threatening septicaemia and thus a spell in intensive care.
There are a small number of reasons for a high white blood cell count (a leukocytosis). These include infection, an autoimmune disease such as lupus, and leukaemia. The latter is relatively easy to diagnose as there is also anaemia and low platelets (thrombocytopenia). It is possible that a preoperative blood sample may suggest an autoimmune disease, but if so there will be other clinical clues and a history. However, a leukocytosis is more than likely to indicate a current, possible low-grade infection. But once again, other blood tests should be informative, and there should also be an increase in CRP, the ‘gold standard’ marker of inflammation. If the leukocytosis does indeed suggest an infection, then postponing the surgery should be considered and the patient should be placed on antimicrobial therapy.
White blood cells do not have a great part to play during surgery. However, they are likely to be primed by the operation, as in the acute phase response.
After surgery
One of the most common and potentially life-threatening consequences of surgery is infection. As part of the acute stress response associated with surgery, the body rapidly mobilises ‘dormant’ white blood cells, and places them in the blood, ready in case of an infection. This is also associated with other changes, such as a rise in the CRP. Thus a modest increase in the white blood cell count after surgery does not necessarily imply an infection. However, if a leukocytosis is more marked (perhaps 12–15 x 109/L) and persistent (exceeding three to four days), then an infection will seem more and more likely.
Ideally, a leukocytosis, either as part of the acute phase response or a genuine response to an actual infection, will eventually fall. However, a persistent leukocytosis may be the consequence of an acute inflammation becoming transformed into chronic inflammation. If so, then a different class of chemotherapy may be called for.
Coagulation and surgery
The coagulation system exists to minimise blood loss and it does this by the controlled formation of a thrombus, formed from platelets and the blood protein fibrin (see Figure 10.3, page 196). Both fibrin and platelets are necessary to form a clot and an excess of either one cannot make up for a lack of the other. However, part of the normal and healthy acute phase response is an increased tendency to clot, which, if extensive, can be fatal. In such a case, prevention is essential.
Prior to surgery
It is necessary to ensure that the patient has a coagulation system that is ‘capable’ of functioning to minimise blood loss. A clear history of previous bleeding problems at the time of surgery, either personally for the patient or from the patient’s blood relatives, needs to be elucidated, coupled with any evidence that the patient has a bleeding tendency by bruising easily or has prolonged bleeding when sustaining abrasions or cuts.
Provided that the patient is not on any antithrombotic treatment such as warfarin, the key result with such a patient initially relates to the platelet count, with further investigation of the level of fibrinogen, INR and PTT ratio. The latter two will give an indication of the workings of the two different aspects of the coagulation system. Patients with deficiencies in this pathway may be unable to form a protective thrombus and so may haemorrhage. Ideally, one would not normally operate on such patients, but life-threatening emergencies may take precedence.
Thrombocytopenia slightly below the bottom of the reference range (150 x 1012/L) may be acceptable if the surgery is minor and blood loss is expected to be minimal. However, if the thrombocytopenia is more profound (75–125 x 1012/L) then a transfusion of a platelet concentrate may be appropriate, and if less than 50 x 1012/L, transfusion would be mandatory. However, discussion between the haematologist and the surgeon and anaesthetist is important to address the risks of proceeding to surgery. The cause of the thrombocytopenia should alsobe determined before surgery, as this may have an impact on postoperative care. For example, in the case of immune-mediated thrombocytopenia, mediated by an autoantibody to platelets, immunosuppression with an agent such as prednisolone may be advisable.
A similar situation is present in the face of low levels of fibrinogen (possibly less than 1.0 g/L) – is the surgery really necessary, and if so, how can we improve the haemostasis of the patient? Once more, one option is a transfusion of fresh frozen plasma (FFP), which will contain fibrinogen as well as other coagulation proteins.
After surgery
A major risk of surgery is haemorrhage, and the body has its own defence system ready to cope should this occur – an increased tendency to form a clot, generally in veins. This is called venous thromboembolism, and its occurrence is a veno-thromboembolic event (therefore both abbreviated to VTE). When this happens in veins of the leg it is called a deep vein thrombosis (DVT), whereas when it happens in a vessel in the lungs it is called pulmonary embolism (PE). Not only do DVT and PE lead to considerable morbidity; clots also kill!
• A DVT leads to a swollen, painful leg such that walking can be difficult, perhaps impossible. DVTs by themselves are rarely fatal but can produce considerable long-term morbidity. There is also powerful evidence that DVT leads to PE. About two-thirds of all VTEs are DVT.
• Blockage of a crucial lung vessel (be it an artery or a vein) by a PE will lead to breathlessness and pain, can lead in the long term to congestive lung disease and heart disease, and can indeed be fatal. PEs make up about a third of all VTEs.
But how big a problem is VTE and who is likely to suffer an event? According to the House of Commons Health Committee,2 about 10% of hospital deaths (1% of admissions) were attributable to PE. However, lower limb DVT has been documented in 50% of major orthopaedic operations performed without anti-thrombotic prophylaxis, in 25% of patients with acute myocardial infarction and in more than 50% of acute ischaemic stroke cases.
The risk of VTE after major general surgery has been extensively documented. Risk factors include abdominal or thoracic surgery requiring general anaesthesia of over 30 minutes. However, lower extremity orthopaedic operations such as total hip and knee replacement carry a particularly high risk and, without prophylaxis, about 50% develop VTE. Arthroscopy is particularly low risk, so prophylaxis is optional, depending on other risk factors. VTE is common in fracture of the pelvis, hip or long bones. Indeed, in one of the first trials of an anticoagulant, the incidence of death from PE after hip fracture fell from 10% to zero!
However, the patient may have other risk factors unrelated to surgery, such as obesity (body mass index >30), age over 60 years or cancer. Not all these risk factors and types of surgery are as dangerous as others. Depending on the risk of the different types of surgery, and on different risk factors that the patients have, they will be started on an anticoagulant. This is likely to be one of the several types of low molecular weight heparin (LMWH), such as enoxaparin, or oligosaccharide anticoagulant such as fondaparinux, and some patients may be moved on to warfarin.
Prevention and treatment of venous thromboembolism and surgery
In view of the high incidence of venous thromboembolism in the perioperative period several guidelines have been developed over the last two decades to try to reduce their incidence and also to reduce the morbidity and mortality associated with them. The guidelines identify several modalities including anticoagulant therapy as prophylaxis to be used in different clinical settings to prevent venous thromboembolism.
• Perhaps the most accessible is the British National Formulary (BNF),3 widely available in NHS hospitals and regularly updated. See http://www.bnf.org
• The British Committee on Standardisation in Haematology (BCSH) offers reasonably up-to-date guidelines for outpatient treatment of DVT with warfarin or heparin at http://www.bcshguidelines.com.
• The UK national guideline-setting body, National Institute for Health and Clinical Excellence (NICE), has released its Guideline 46, devoted to reducing the risk of thrombosis after surgery. This is available at http://www.nice.org.uk
• The National Patient Safety Agency (NPSA) has released its own guidelines on the management of the patient on warfarin, available at http://www.nrls.npsa.nhs.uk/
Local practice and management
Each healthcare organisation should have a ‘thrombosis committee’ to develop and issue local guidelines for the prevention of venous thromboembolism. In the UK this will be based on the NICE Guideline 46 released in April 2007, as well as the guidance from the NPSA related to the management of patients on warfarin. Each practitioner should gain access to their local guidelines for assessing the risk of each patient and ensure that the patient receives the recommended appropriate modality in their organisation in collaboration with other members of the multi-disciplinary team. Coupled with this is the need to give both verbal and written information to the patient about the risks of VTE and the effectiveness of prophylaxis.
Statement
What follows is informed comment and NOT guidelines.
NO responsibility is taken for their use in clinical practice.
Practitioners are expected to refer to their own Hospital Guidelines.
NB: Consider NICE Guideline 46.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





