Chapter 13 The Process of Organ Donation and Pediatric Donor Management
Overall, the demand for organs continues to increase at a rapid pace, far exceeding the number of organs available for transplantation. Approximately 2% of the patients on the national transplant wait list are children 18 years of age or younger.1 However, in contrast to the increasing number of adults on the wait list, the number of children has decreased during the past several years. The reason for this decrease in large part is because more organ transplants are occurring in children.1 Additionally, patients in need of a transplant who in the past would have died from organ failure while waiting are now living longer lives as a result of improved pharmacologic and medical management. Some of these patients may not require a transplant at all, further reducing the number of children on the national wait list. Nonetheless, children continue to die while waiting for a life-saving transplant, with the highest death rate observed in children younger than 1 year.1 Additionally, another 40 to 50 children are removed from the pediatric wait list annually because their condition has deteriorated to a degree where transplantation is no longer an option.1 The need for more organs is clear in this patient population.
The number of adult donors has continued to increase during the past 10 years, while the number of pediatric donors has decreased.1 This decline in pediatric donors has occurred for many reasons, including improved medical and surgical treatments, vaccinations that have reduced deaths associated with childhood infectious diseases, safety restraints, education and awareness regarding health hazards affecting children, and pediatric critical care specialists who have reduced morbidity and mortality for children who are critically ill or injured.
Missed opportunities for organ donation occur in many medical institutions nationally.2 Frequently these losses occur when families decline the option of donation, but in some cases families are not even being given the opportunity for donation. Organs available for transplantation may be lost because of caregivers’ lack of familiarity with appropriate donor management, or opportunities for donation may be denied by medical examiners who fear losing evidence and the ability to successfully prosecute homicide cases in situations where abusive trauma has claimed the life of a child.
The process of donation begins when a critically ill or injured child is identified as a potential donor with a timely referral to the organ procurement organization (OPO). Early involvement of the OPO enhances chances of recovering viable organs for transplantation while allowing coordination of the donation process. Medical management of the potential pediatric organ donor requires knowledge of the physiologic derangements associated with this patient population. Hemodynamic instability, alterations in oxygenation and ventilation, metabolic and endocrine abnormalities, and coagulation disturbances are common. Support and care of the family provided by a team of physicians, nurses, social workers, chaplains, and other support staff trained in the unique aspects of pediatric medicine are integral to the care of these children and their families.3,4 Each of these elements is essential to ensure successful recovery of organs from this select group of patients.
Role of the Pediatric Intensivist and Critical Care Team in the Process of Organ Donation
Effective donor management is crucial to the successful recovery of organs for transplantation. The integral involvement of pediatric critical care specialists in the management of critically ill and injured children has been a foundation of the clinical practice of critical care in many successful pediatric centers. Caring for the critically ill child and his or her family through all phases of illness, including end-of-life issues, should be a seamless transition. The continuum of care for the dying patient who progresses to death and becomes a donor requires the expertise of the pediatric intensivist and critical care team to manage and prevent deterioration of organ systems and loss of transplantable organs. Involvement of critical care specialists, especially in pediatrics, where there is a limited and decreasing number of donors,1 improves the quality and number of organs recovered.
It is federally mandated to inform the OPO of any impending death in a timely manner. Suitability of the potential donor needs to be determined and issues regarding donation need to be discussed with the medical team and the family. The model utilizing OPO coordinators and designated requestors to obtain consent from families has been successful in the adult arena; however, we continue to learn that the situation with children and their families is different. One study found that parents seem to prefer discussions regarding donation with the pediatric intensivist or a member of the health care team they have come to trust, rather than the OPO coordinator.5 Timing of the first request relative to discussions of brain death did not influence the decision to donate organs, but consent for donation was more likely when parents had sufficient time to discuss this issue.5 These concepts are vastly different from the traditional approach in which the OPO coordinator requests consent and decouples the death and consent process. Improved communication between the health care team and the OPO remains important, however. A team approach has been adopted by many institutions for discussing donation with families. This practice is encouraged in the United States Department of Health and Human Services Organ Donation Collaborative’s final report on best practices.6 Best practices promote coordinated efforts between OPO coordinators and medical professionals to improve consent rates while assisting families with end-of-life care issues. Involvement of palliative care teams also has been identified as another resource to assist the intensive care unit (ICU) team, parents, and families facing end-of-life issues with their child.
Determination of Brain Death
The “dead donor rule” states that patients must be declared dead before removal of vital organs for transplantation can occur.7 Therefore, accurate determination of brain death is essential before efforts at organ recovery proceed. Determination of brain death must occur in a timely and efficient manner for several reasons: it allows the family to begin the grieving process as they prepare for the loss of a loved one; it allows the process of organ preservation and preparation for recovery to begin; and if donation is not planned, medical therapies can be stopped, allowing redistribution of scarce ICU resources to other critically ill and injured patients.
Good donor management is imperative for recovery of viable organs for transplantation. Hemodynamic instability and organ dysfunction account for a loss of up to 25% of potential donors when donor management is not optimized.8 Furthermore, the institution of hormonal replacement therapy (HRT) early in the donation process may assist with stabilization of the donor, improve the quality of organs recovered, and enhance posttransplant graft function.9–13
The determination of brain death in children is a clinical process based on clinical criteria that are consistent across the age spectrum. No unique legal issues differentiating declaration of brain death exist for children. However, age-related issues can make determination of irreversible injury and declaration of brain death more difficult in younger patients, resulting in age-based recommendations.14 The clinical history, cause of coma, and brain injury must be determined to ensure that an irreversible condition has occurred. Physical examination criteria rely upon the coexistence of coma and apnea in a child who is neither hypothermic nor hypotensive for age and whose examination is not affected by sedatives or neuromuscular blocking agents. Neurologic criteria to determine brain death in infants and children are listed in Box 13-1. Absence of neurologic function is defined by the following features on physical examination: mid-position and fully dilated nonreactive pupils, absence of spontaneous eye movements induced by oculocephalic or oculovestibular testing, absence of bulbar function, cough, corneal, gag, and rooting reflexes, and absence of respiratory effort when challenged with elevated carbon dioxide levels that otherwise would induce respiratory effort in such a patient. Serial neurologic examinations are necessary to establish the diagnosis of brain death. The duration of observation between examinations and the need for ancillary studies is based on age, history, and clinical examination. The examination results must remain consistent with brain death throughout the observation and testing period.
Box 13–1 Neurologic Examination Criteria for Brain Death in Children
Data from American Academy of Pediatrics Task Force on Brain Death in Children: Report of Special Task Force: guidelines for the determination of brain death in children, Pediatrics 80:298-300, 1987.
Determination of brain death for any infant or child requires establishing and maintaining normal physiologic parameters. Before neurologic examination or neurodiagnostic testing for brain death can be meaningful, correction of hypotension and hypothermia must occur. The 1987 guidelines for determination of brain death in children provided no definition of hypothermia.14 Current adult and Canadian guidelines use a core body temperature of >35° C (95° F).15,16 Conditions that can interfere with the neurologic examination or factors capable of imitating brain death must be excluded. Conditions such as severe hepatic or renal dysfunction, inborn errors of metabolism, or metabolic disturbances may play a role in the clinical presentation of the comatose infant or child. These conditions should be considered and, if identified, appropriate treatment should be instituted to correct the derangements resulting in coma. Instances may occur in which these conditions cannot be corrected and additional ancillary testing may be required to confirm brain death. Testing for drug intoxications including barbiturates, opiates, and alcohol should be performed as indicated. The half-life of sedative agents must be considered when determining the appropriate timing of the clinical examination. Longer acting or continuous infusion of sedative agents and recent administration of neuromuscular blocking agents can interfere with the neurologic examination. These agents should be discontinued for a reasonable amount of time to allow adequate clearance of the drug(s) prior to initiating electroencephalogram (EEG) testing and clinical examination for brain death testing. Barbiturates reduce cerebral blood flow (CBF); however, no evidence exists that high-dose barbiturate therapy completely arrests CBF. Evidence suggests that radionuclide CBF study or cerebral arteriography can be utilized in patients who have undergone high-dose barbiturate therapy to demonstrate absence of CBF.17,18 Clearance of neuromuscular blockers can be confirmed by use of a nerve stimulator.
The apnea test is a critical and essential component of the clinical examination to determine brain death. Testing for apnea must allow adequate time for the partial pressure of carbon dioxide, arterial (PaCO2) to increase to levels that would normally stimulate respiration. Apnea testing must be performed while maintaining normal oxygenation and stable hemodynamics. Patients should be preoxygenated with 100% oxygen to prevent hypoxia. Mechanical ventilatory support should be adjusted to eliminate ventilation, allowing the carbon dioxide tension (PCO2) to rise while observing the patient for spontaneous respiratory effort. False reports of spontaneous ventilation have been reported while patients were maintained on continuous positive airway pressure for apnea testing despite having sensitivity of the mechanical ventilator reduced to minimum levels.19 Attaching a self-inflating bag valve system, such as a Mapleson circuit, to the endotracheal tube or tracheal insufflation of oxygen using a catheter inserted through the endotracheal tube also has been used to provide supplemental oxygen. High gas flow rates with tracheal insufflation may promote carbon dioxide washout, preventing adequate PaCO2 rise during apnea testing. Additionally, adequate gas outflow must be ensured to prevent barotrauma. The PaCO2 should be measured and allowed to rise to 60 mm Hg or greater while continually observing the patient for any spontaneous respiratory movements over a 5- to 10-minute period. If no respiratory effort is observed during this time, the apnea test is consistent with brain death. The patient is reconnected to mechanical ventilator support until death is confirmed with either a repeat clinical examination or an ancillary study. If the apnea test cannot be completed because of hemodynamic instability, desaturation, or an inability to reach a PaCO2 of 60 mm Hg, an ancillary study should be pursued to make the diagnosis of brain death. Infants and children with chronic respiratory disease or insufficiency may only breathe in response to supranormal PaCO2 levels. In this instance, the PaCO2 level should rise to ≥20 mm Hg above their baseline PaCO2 level. If there is any concern regarding the validity of the apnea test, an ancillary study should be pursued.
Recommended clinical observation periods between examinations in children differ from those for adults, with a greater duration suggested for younger children. Box 13-2 lists the recommended observation periods based on the age of the infant or child from the Task Force Guidelines for Brain Death in Children.14 Observation periods have never been validated. Many authors have argued that except in very immature, preterm newborns, the same criteria to declare brain death can be applied to full-term newborns, infants older than 7 days of age, children, and adults.20–27 Despite this controversy, the only published guideline to determine brain death in children recommends observation periods based on the age of the child.14 The Special Task Force for the determination of brain death provided no guidelines to diagnose brain death in infants younger than 7 days of age.14 Guidelines for this age group were not defined because of limited clinical experience, lack of sufficient data, and concerns about the ability to reliably confirm irreversibility of brain injury in this patient population.25,26 This situation does not imply that brain death does not occur in this patient population, only that it can be more difficult to diagnose. Diagnosing brain death can occur in the term infant, even in those younger than 7 days of age; however, an observation period of 48 hours has been recommended to confirm the diagnosis. It has been suggested that the observation period can be reduced to 24 hours if ancillary studies demonstrate no CBF or an isoelectric EEG.26,27 The younger the child, the more cautious one should be in determining brain death.
Box 13–2 Recommended Observation Period to Determine Brain Death in Infants and Children
Data from American Academy of Pediatrics Task Force on Brain Death in Children: Report of Special Task Force: guidelines for the determination of brain death in children, Pediatrics 80:298-299, 1987.
Older than 1 Year
Ancillary studies can provide additional supportive information to assist in declaring brain death. These studies are useful when the clinical examination or apnea testing cannot be safely completed because of the underlying medical condition of the patient, if there is uncertainty about the findings of the neurologic examination, or if a confounding medication effect may be present. Ancillary studies are not necessary if determination of brain death can be made based on clinical examination criteria in the older child. They are, however, recommended by the Task Force for children younger than 1 year.14 Ancillary studies may be utilized to expedite the diagnosis of brain death by reducing the clinical observation period, potentially increasing the viability of transplant tissue. However, in the circumstance that an ancillary study is equivocal, the observation period can actually be increased until another study or clinical examination is performed to confirm brain death. Ancillary studies can be helpful for social and medical reasons. These studies may allow family members to better comprehend the diagnosis of brain death and may be important in situations where death is the result of homicide. Ancillary studies are not a substitute for a complete physical examination.
Four-vessel cerebral angiography evaluating anterior and posterior cerebral circulation remains the gold standard to determine blood flow for brain death testing; however, this test is difficult to perform in small children and requires technical expertise that may not be available in every facility. Furthermore, transporting a potentially unstable patient to the angiography suite carries additional risk that can complicate this process. For these reasons, cerebral angiography is rarely performed. EEG documentation of electrocerebral silence and absence of CBF using radionuclide CBF study remain the most widely available and useful ancillary studies to assist with the diagnosis of brain death in infants and children. These studies are more easily accomplished at the bedside, without the need for extraordinary technical expertise. Radionuclide CBF studies have been used extensively with good experience.28 Use of the portable gamma camera for radionuclide angiography has made CBF studies more accessible, allowing for the study to be undertaken at the bedside.28,29 This study is becoming a standard in many institutions, replacing EEG as an ancillary study to assist with the determination of brain death in infants and children.30 EEG and radionuclide CBF studies are both accepted ancillary studies used to assist the clinician in determining brain death in children. EEG may be more specific, although less sensitive, than the radionuclide CBF study. EEG testing evaluates cortical and cellular function, while radionuclide CBF testing evaluates flow and uptake into brain tissue. Each of these tests requires the expertise of appropriately trained and qualified individuals who understand the limitations of these studies to avoid misinterpretation. Transcranial Doppler sonography and brain stem audio-evoked potentials have been utilized29,31 but have not been studied extensively or validated in children.25,32,33 These studies, along with computed tomography angiography, perfusion magnetic resonance imaging, and magnetic resonance angiography-magnetic resonance imaging cannot be relied upon as dependable ancillary studies at this time.32,33
Ancillary studies are least sensitive in the neonatal age group.26,34 Limited experience with ancillary studies performed in the newborn younger than 30 days of age indicates that EEG is less sensitive than CBF in confirming the diagnosis of brain death. Sensitivity remains quite low, however, even with CBF, for this age group.25,27,34,35 Diagnosing brain death in the neonate can be more difficult; therefore, serial examinations are essential to ensure that the clinical examination remains consistent throughout the observation and testing period. The younger the child, the more cautious one should be in diagnosing brain death. If there is any uncertainty about the examination or the ancillary study, continued observation and clinical examination or a repeat ancillary study should be performed to make the diagnosis of brain death.
Diagnosing brain death has great implications with profound consequences. The clinical diagnosis of brain death is highly reliable when made by experienced examiners using established criteria.36,37 There are no reports of children recovering neurologic function who met adult brain death criteria upon neurologic examination.35 Diagnosis must never be rushed or take priority over the needs of the patient or the family. Appropriate emotional support for the family should be provided, including adequate time to grieve with their child after death has occurred. Each state has laws or regulations for determination of death that have, in most cases, been modeled after the Uniform Determination of Death Act.38 The Uniform Determination of Death Act states that a person who has sustained either (1) irreversible cessation of circulatory and respiratory functions or (2) irreversible cessation of all functions of the entire brain, including the brain stem, is dead. A determination of death must be made in accordance with accepted medical standards. The reader is encouraged to become familiar with guidelines in his or her institution. At the time this chapter was being written, guidelines for the determination of brain death in infants and children were being reviewed and revised by a committee formed by the Society of Critical Care Medicine and The American Academy of Pediatrics.
Brain Death Physiology
As the patient with severe intracranial pathology progresses to brain death, neuroendocrine dysfunction often requires specific interventions. These physiologic derangements reflect the powerful neuroendocrine changes that occur during progression to brain death. Efforts to control cerebral perfusion pressure, hemodynamic manifestations of herniation, and loss of central nervous system function all contribute to the instability that routinely occurs during and after progression to brain death. These physiologic changes clearly affect end-organ viability in the prospective organ donor. Understanding the physiologic changes and anticipating associated complications with brain death therefore is critical for organ recovery.
Loss of central nervous system function causes diffuse vascular regulatory and cellular metabolic injury.39 Brain death and cerebral ischemia increase circulating cytokines,40 reduce cortisol production,41 and precipitate massive catecholamine release. The combination of these factors results in physiologic deterioration and ultimately end organ failure if left untreated.
CBF is approximately 50 mL/100 g/min and consumes 15% of the cardiac output.42 Without consumption by the brain, glucose needs are reduced and the patient is prone to hyperglycemia. Furthermore, with this reduction in cerebral metabolism, carbon dioxide production falls, resulting in a reduction in PaCO2. Hypothermia should be anticipated as a result of hypothalamic failure and loss of thermoregulation. Additionally, impaired adrenergic stimulation results in loss of vascular tone with systemic vasodilation and increased heat loss. Neuroendocrine dysfunction occurs because of inhibition or loss of hormonal stimulation from the hypothalamus, resulting in fluid and electrolyte disturbances and eventually cardiovascular collapse if left untreated.
Hemodynamic deterioration that occurs with brain death is initiated by a massive release of catecholamines, commonly referred to as sympathetic or autonomic storm, associated with cerebral ischemia and intracranial hypertension. This deterioration manifests clinically with systemic hypertension and tachycardia.39,43 During this autonomic storm, organs are exposed to extreme sympathetic stimulation from direct neural stimulation or from significant increases in endogenous catecholamines. The local effects of such sympathetic stimulation include increased vascular tone, effectively reducing blood flow and potentially causing ischemia to these organs.
This autonomic storm also has direct effects on the myocardium as the surge of catecholamines increases systemic vascular resistance, myocardial work, and oxygen consumption.44 This imbalance between myocardial oxygen supply and demand39,45 can cause ischemic changes. Myocardial injury can impair cardiac output and lead to dysfunction of other organs. Left ventricular end diastolic pressure rises, causing pulmonary edema. This condition may be exacerbated by displacement of systemic arterial blood into venous and pulmonary circulations as a result of catecholamine-mediated systemic vasoconstriction. Increased pulmonary vascular resistance and right heart volume overload may displace the ventricular septum into the left ventricle, further impairing cardiac output by impeding left ventricular filling.46
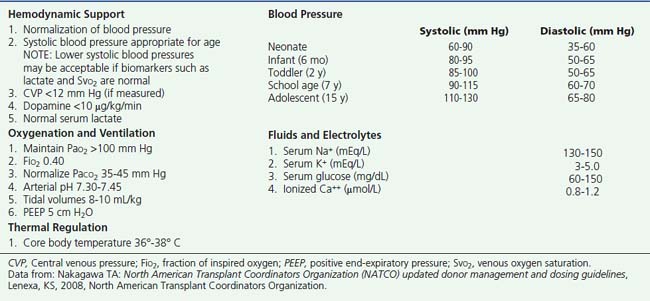
Following determination of brain death, and once a decision has been made by the family to proceed with organ donation, the focus of care shifts toward the preservation of vital organs. Subsequent care may differ from management before death. Efforts to reduce intracranial pressure are abandoned and care shifts toward providing ample blood flow and oxygen delivery to transplantable organs. Hemodynamic management goals are directed at maintaining normal peripheral perfusion and blood pressure for age. Decreased intravascular volume, caused by efforts to reduce CBF and control intracranial pressure (e.g., volume restriction and diuretic agents), must be corrected. Attention to volume loss from derangements such as diabetes insipidus (DI), must be anticipated and addressed. Additional donor management goals include the normalization of PCO2, normalization of temperature, and correction of metabolic disturbances. Donor management goals are listed in Box 13-3. Progression from brain death to somatic death and loss of transplantable organs can result if appropriate care is not instituted.8,46 Aggressive donor management optimizes organ function and affects the quality of organs recovered.11,46 This scenario can result in more transplantable organs and improved graft function,9–12 potentially reducing the length of the hospital stay and decreasing the incidence of morbidity and mortality for the transplant recipient.
Box 13–3 Pediatric Donor Management Goals
Data from: Nakagawa TA: North American Transplant Coordinators Organization (NATCO) updated donor management and dosing guidelines, Lenexa, KS, 2008, North American Transplant Coordinators Organization.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree