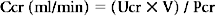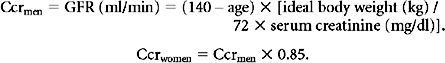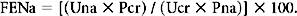CHAPTER 88 THE MANAGEMENT OF RENAL FAILURE: RENAL REPLACEMENT THERAPY AND DIALYSIS
Acute renal failure (ARF) is a common and devastating problem that contributes to morbidity and mortality in critically ill patients. ARF prolongs hospital stays and increases mortality. Although effective renal replacement therapy (RRT) is available, it is not ideal and the best therapy is prevention.
INCIDENCE
Acute renal failure is defined as an abrupt and sustained decline in the glomerular filtration rate (GFR),1 which leads to accumulation of nitrogenous waste products and uremic toxins. In critically ill patients, more than 90% of the episodes of ARF are due to acute tubular necrosis (ATN) and are the result of ischemic or nephrotoxic etiology (or a combination of both). ARF affects nearly 5% of all hospitalized patients and as many as 15% of critically ill patients.2 Like many other medical conditions, there is no gold standard of diagnosis, no specific histopathologic confirmation, and no uniform clinical picture.
The mortality rate of an isolated episode of ARF is approximately 10% to 15%. When it occurs in association with multiple-organ dysfunction, as in the ICU setting, mortality rates are much greater and vary in published series between 40% and 90%.3
In some cases, preexisting conditions may worsen. New major complications, such as sepsis and respiratory failure, may also develop after the onset of renal failure. Although ARF that requires RRT carries a high mortality,4 there is emerging evidence to suggest that milder forms of ARF that do not require supportive therapy with RRT have better patient outcomes.5
MECHANISM OF INJURY/ETIOLOGY
Assessment of Renal Function
Serum concentrations of blood urea nitrogen (BUN) and creatinine are the most commonly used markers of renal function. Urea is the end product of protein and amino acid catabolism. Under normal conditions, 80%–90% of total nitrogen excretion is by the kidneys. Creatinine is formed in muscle by the nonenzymatic degradation of creatine and phosphocreatine, and is excreted primarily by glomerular filtration. A small percentage of creatinine is actively secreted into the glomerular filtrate and tubular reabsorption of creatinine is negligible.5
Creatinine Clearance
where Ucr is urine creatinine, Pcr is serum creatine, and V is volume.
Normal GFR is 125 ± 15 ml/min/1.73 m2 body surface area (BSA).
Here, Una and Ucr are the urinary concentrations of sodium and creatinine, and Pna and Pcr are the serum levels of sodium and creatinine, respectively. If the FENa is very low (<1%), it may indicate inadequate renal arteriolar pressure—suggesting that factors other than intrinsic renal dysfunction are responsible for clinically inadequate renal function.6
Urine Production and Output
The end result of renal function is the production of urine. Quantitative measurements of urine are important for assessing renal function. Urine output is highly sensitive to renal blood flow, making it a key indicator of renal function and total body vascular perfusion7 (Table 1).
| Age | Urine Output (ml/kg/min) |
|---|---|
| Infant (<10 kg) | 2.0 |
| Toddler (10–20 kg) | 1.5 |
| Child (20–50 kg) | 1.0 |
| Adult (>50 kg) | 0.5 |
MANAGEMENT OF PATIENTS
Nonpharmacologic Strategies for Acute Renal Failure Prevention
Fluids
Adequate hydration is the cornerstone of renal failure prevention. One randomized controlled trial (n = 1620) compared hydration using 0.9% saline infusion with 0.45% saline in dextrose for prevention of radiocontrast-induced nephropathy in patients who underwent coronary angiography.8 Hydration with 0.9% saline infusion significantly reduced contrast nephropathy compared with 0.45% saline in dextrose hydration (0.7% vs. 2%, respectively; p = 0.04). This effect was greater in women, diabetics, and patients who received a large volume (>250 ml) of a contrast agent. A recent single-center randomized controlled trial compared the efficacy of sodium bicarbonate with 0.9% saline hydration in preventing contrast nephropathy.9 In this study, 119 patients who had stable serum creatinine of at least 1.1 mg/dl were randomized to 154 mEq/l infusion of sodium chloride (n = 59) or sodium bicarbonate (n = 60) before and after contrast (iopamidol) administration. One of 59 patients (1.7%) in the group that received bicarbonate developed contrast nephropathy (defined as an increase of ≥25% in serum creatinine from baseline within 48 hours) compared with 8 of 60 patients (13.3%) in the group that received saline (p = 0.02).
Nephrotoxin Exposure
Minimizing exposure to potentially nephrotoxic agents is an important strategy to prevent ARF in the ICU setting. Aminoglycosides, other antibiotics, amphotericin, and radiocontrast are the nephrotoxins encountered most commonly in the ICU. A systematic review in patients who had neutropenic fever and received aminoglycosides, however, found no significant differences in efficacy or nephrotoxicity between once daily and three times daily dosing.10
The use of lipid formulations of amphotericin B seems to cause less nephrotoxicity compared with standard formulations, but direct comparisons of long-term safety are lacking. With regard to contrast media, one systematic review (31 randomized controlled trials, 5146 patients) compared low osmolality contrast media with standard contrast media.11 The study showed that low osmolality contrast media did not influence the development of ARF or the need for dialysis.
Pharmacologic Strategies for Acute Renal Failure Prevention
Loop Diuretics
Multiple small clinical trials studied the efficacy of loop diuretics in preventing ARF and have provided conflicting results. They have been underpowered, nonrandomized, or methodologically flawed. One systematic review that compared fluids with diuretics in people who were at risk for ARF from various causes did not show any benefit from diuretics with regard to prevalence of ARF, need for dialysis, or mortality.12
N-Acetylcysteine
Systematic reviews found that NAC plus hydration reduced the incidence of contrast nephropathy more than hydration alone in people who had baseline renal impairment and underwent radiocontrast studies.13 A recent study, however, suggested that NAC could decrease serum creatinine independently without any effect on GFR (as evaluated by other surrogate outcomes, such as serum cystatin C levels).14 Hence, the current implications of reduction in serum creatinine after contrast administration with the use of NAC remain unclear and need to be explored further.
INDICATIONS FOR RENAL REPLACEMENT THERAPY IN ACUTE RENAL FAILURE
As in chronic kidney disease, overt disturbances of ECF volume and body fluid composition remain the objective indications for initiation of RRT in patients with ARF (Table 2). These include volume overload, hyperkalemia, severe metabolic acidosis, uremia, and azotemia.
Table 2 Indications for Renal Replacement Therapy
Volume Overload
Mehta and colleagues15 performed a retrospective analysis of data from 522 critically ill patients who had ARF. Fifty-nine percent of these patients had been treated with diuretics. After adjustment for relevant covariates and the propensity for diuretic use, they observed a significant increase in the risk of death or nonrecovery of renal function (odds ratio 1.77, 95% confidence interval 1.14–2.76). On the basis of this, they concluded that diuretic therapy was potentially deleterious in patients who had ARF. They noted, however, that the increased risk was borne largely by patients who were unresponsive to diuretics. This suggested that this increased risk might reflect selection for a more severe degree of renal injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree