Fig. 49.1
Henry K. Beecher (1904–1976). (Courtesy of the Wood Library-Museum of Anesthesiology, Park Ridge, IL.)
Premedication is part of the history of anesthesia and thus deserves inclusion in our story. But it is more than part, more than a recitation of the drugs used at various times. Premedication responded to the anesthetics used, the evolution of anesthetic practice (e.g., the elimination of the preanesthetic visit; the shift to outpatient anesthesia), a greater focus on patient demands, and an increasing knowledge of drug pharmacokinetics and dynamics. As such the history of premedication also reflects the history of anesthesia.
Ancient Times
Prior to the discovery of anesthesia, alcohol and opium might be given before surgery,. These could not substitute for anesthesia, but provided some comfort. Wedel suggested that “Opium in a moderate draft (might be given) to the patient on the night preceding the operation (amputation), for thus he bears the burning and cutting of the limb with a readier spirit, and various symptoms will be averted” [3]. But this could hardly be called premedication in anticipation of anesthesia. The controlled administration of drugs before the induction of anesthesia’demanded the invention of a precision means of delivery, the hollow needle, invented by Francis Rynd in 1844, and the hypodermic syringe, introduced by Charles Pravaz in 1853 [4].
Premedication: The Ether and Chloroform Era
Induction of ether anesthesia is slow. Its pungency can cause coughing, breath holding and laryngospasm (closure of the larynx). It stimulates salivary secretions which, in turn, add to the potential for coughing, breath holding and laryngospasm. An inhalation induction of anesthesia with ether has one other drawback’it can be frightening, invoking a sense of claustrophobia and suffocation, a feeling of swirling down into a black hole. One of the editors (LJS) has never forgotten two terrifying childhood experiences’in each being restrained while desperately trying to breathe in the face of ether’s pungency. He heard a buzzing sound that increased to a crescendo in association with visual hallucinations’until unconsciousness blessedly intervened. Administration of a drug producing anxiolysis might have had a salutary effect on such fears. Anesthetists often ignored some of these untoward effects for a century, effects that should have mandated the use of drugs to speed induction, decrease the perception of pungency, decrease anxiety, and prevent secretions.
Over the last half of the nineteenth century clinicians increasingly injected morphine and scopolamine or atropine before ether anesthesia, scopolamine and atropine minimizing the production of secretions [5,6]. We know from modern studies that premedication with morphine (and the siblings of morphine’opioids such as fentanyl) can minimize some of the problems noted in the preceding paragraph. Premedication with a standard dose of morphine decreases anesthetic (i.e., ether) requirement by approximately 10% [7]. That is, premedication with morphine provides a head start in the induction process. It also decreases the perception of pungency. Premedication with morphine or fentanyl decreases the incidence of coughing on induction of anesthesia with desflurane, an inhaled anesthetic that, like ether, is pungent (Fig. 49.2) [8].
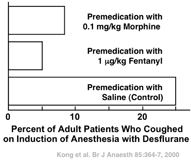
Fig. 49.2
Premedication with either morphine or fentanyl decreases the incidence of coughing on induction of anesthesia with the pungent anesthetic desflurane by 65 to 80%. (From Kong CF, Chew STH, Ip-Yam PC: Intravenous opioids reduce airway irritation during induction of anaesthesia with desflurane in adults. Br J Anaesth 2000; 85: 364–7, with permission.)
Chloroform presents different problems. It is more potent and less pungent than ether, but it can induce premature ventricular contractions, especially in the anxious patient. Even with chloroform, the need to facilitate the induction of anesthesia remained. As with ether, morphine and atropine evolved as the premedication drugs most commonly selected for patients about to receive chloroform.
Premedication might be used for particularly difficult patients. Carl Uterhart (1835–1895)
“…observed that the onset of the chloroform narcosis, which in alcoholics is known to be preceded by a severe and dangerous state of excitement, was extremely easy…after a small dose (of morphine). The state of excitement failed to appear; a quiet sleep with complete relaxation of all muscles occurred immediately after use of a few drams of chloroform” [9].
And we admire the advice of Joseph Clover, who in 1874 said
“I like to give a teaspoonful of brandy, without water, a few minutes beforehand, but not so much as a tablespoonful. If wine be given or if the patient must have some water in the brandy, then they should be given half an hour before inhaling, to allow time for absorption” [10].
In 1881, Henry Lyman gave similar advice for care preceding anesthesia with ether [11]:
“…there are many nervous individuals who cannot contemplate the approaching danger of operation without the greatest degree of agitation. Every possible effort should be made to calm such apprehensions. The tact of the surgeon will guide him to the methods best calculated to effect his purpose in this particular. The administration of a few doses of an alcoholic stimulant before inhalation is highly recommended as a means of tranquilizing a timid patient.”
In 1850, Lorenzo Bruno of Turin (Fig. 49.3) reported injection of morphine 1 hour before induction of chloroform anesthesia to “lessen psychic trauma” [12]. Claude Bernard (Fig. 49.4), Professor of Physiology at the Sorbonne had worked in Bruno’s clinic. In 1869, Bernard gave a series of lectures at the College de France describing the injection of morphine into animals to decrease the amount of chloroform needed for deep anesthesia [1,13]. This led Labbé and Guyon to use morphine before chloroform anesthesia in humans, and in 1872 they described their experiences in four patients to the Académie des Sciences [14]. In the same year, Demarquay presented to the Académie des Sciences, his experience giving morphine before chloroform anesthesia [15]. He concluded that this practice added to the dangers of anesthesia and should not be done, but did not indicate the basis for his conclusion. Perhaps he considered that morphine’s ability to depress breathing and thereby induce hypercapnia and hypoxia might lead to cardiac dysrhythmias in the presence of chloroform. Despite these explorations with premedication, its use was apparently not widespread before 1900.

Fig. 49.3
Lorenzo Bruno (1821–1900). (Courtesy ofhttp://www.comune.murazzano.cn.it/Contatti/tabid/1146/Default.aspx,“L’illustrazione italiana ”, Milano, Fratelli Treves, Bibl. Sen. 308/37 p. 356.)

Fig. 49.4
Claude Bernard (1813–1878). (Courtesy of the Wood Library-Museum of Anesthesiology, Park Ridge, IL.)
In her review of developments in the last half of the nineteenth century, Duncum noted some peculiar beliefs regarding the combination of morphine and a belladonna drug, views that continued to the time (1956) when one of the editors (EIE) was a resident [16]. The view was that each drug counteracted the untoward effect of the other drug. For example, giving atropine or scopolamine would prevent the vomiting often associated with morphine. But some data suggested otherwise [17].
The 1880s to the 1950s
Like morphine, using atropine as part of the premedication has a long history. Professor Dastre, a French Physiologist who had worked with Claude Bernard, recognized that atropine prevented chloroform-induced vagal slowing of the heart [1]. In 1878 he described the use of atropine in his animal experiments, conducted under morphine narcosis and a small dose of chloroform [18]. Dastre could not interest Paris surgeons in his method but Aubert, a surgeon from Lyons, embraced Dastre’s suggestions and described giving atropine 0.5 mg with morphine 10 mg to his patients 20 to 30 minutes before chloroform anesthesia [19]. He viewed its advantages as safety, rapid induction, quietness of the patient and reduced postoperative sickness.
In 1880, E.A Schäfer wrote that
“It is well known that atropin paralyses the cardiac inhibitory apparatus, and since it is probable that death in these (chloroform anesthesia) cases results from a stimulation of this apparatus…there undoubtedly seems good reason for the employment of atropin. But clearly, it should be given immediately before the administration of the chloroform, as a preventive…” [20].
Despite such advocacy, European surgeons were slow to embrace the use of premedication. There was also little enthusiasm in Britain. G Cockburn Smith investigated the action of hyoscine in France. In a letter to the Lancet in 1891, he wrote “…the extreme kindness of the (French) surgeons has enabled me to satisfy myself that the dangers of ether and chloroform can be greatly, if not entirely, removed by the injection of one centigramme of hydrochlorate of morphia and one milligramme of sulphate of atropia some twenty five minutes previously to their administration in adults” [21]. Smith’s advice fell on deaf ears.
In 1900, Schneiderlin, of Emmendingen, Germany, introduced the combined injection of morphine and hyoscine prior to anesthesia. He had previously used the combination as a sedative to treat mental patients. He recommended small doses repeated several times, beginning sometimes on the day before operation. However it was the recommendations of Carl Hartog, who in 1903, advised routine administration half to three quarters of an hour before ether, that encouraged the increasing use of premedication in Europe.
In England, Dudley Buxton was the first to promote the use of morphine and hyoscine. In 1909, he wrote
“…a terrified patient after a sleepless night is in the worst condition for an anaesthetic and an operation. In such patients, I am convinced that the use of scopolamine and morphine injections before a general anaesthetic is valuable” [22].
The first enthusiasm for morphine and scopolamine premedication in the US arose in 1910. Clifford Collins of Peoria, Illinois reported on more than 1000 cases:
“Tablets are obtained containing a combination of scopolamin, 1/100 grain (0.065 mg), and morphin 1/6 grain (11 mg), and the solution is made just before it is administered hypodermically, which is done one and a half hours before the operation is begun…all necessary manipulations and handling of the patient in the preparation are completed before the hypodermic is administered. The room is darkened and everything is kept quiet, and he falls into a tranquil slumber. About twenty minutes before the operation, a layer of damp cotton is placed over the eyes and the patient is taken to the operating room and placed on the operating table…” [23].
A Sea Change Should Have Resulted from the Use of Intravenous Barbiturates and Less Soluble, Less Pungent Inhaled Anesthetics
In the mid-1930s, Waters’ group [24] and Lundy and Tovel [25] reported that thiopental (Pentothal®), a short-acting intravenous anesthetic circumvented the claustrophobia and irritant effects associated with induction of anesthesia with ether. Anesthesia now could be induced quickly (“Count backwards from 100, please”) and pleasantly. And in the 1920s and 30s, non-pungent inhaled anesthetics such as ethylene [26] and cyclopropane [27] were introduced into clinical practice. They were wonderful except that they exploded when touched by a spark. Still, just two or three breaths of cyclopropane could induce anesthesia.
Thus, the need for morphine and atropine before anesthesia was much diminished, perhaps gone. However, these discoveries of intravenous and non-pungent inhaled anesthetics did little to alter patterns of use that persisted in spite of diminished pharmacological need. Inertia is indeed a powerful force.
Halogenated Anesthetics and the Need for Routine Morphine Premedication
Fluorinated volatile anesthetics initially appeared in the early 1950s, presenting new pharmacologic characteristics (more potent, more rapid awakening, less airway irritation and fewer associated secretions). This altered the potential need for morphine and atropine. In 1951, RT Stormont, Secretary of the American Medical Association observed [28]:
“Narcotic drugs are often employed more or less routinely for preanesthetic medication without a full appreciation of their relative value for this purpose. The Council (Council on Pharmacy and Chemistry of the American Medical Association) has authorized publication of the following report with the view of encouraging additional well-controlled and critical studies in this and related fields.”
The report referred to by Stormont was: “Narcotics in Preanesthetic Medication” and the authors were Ellis N. Cohen and Henry K. Beecher (Fig. 49.1) [28]. These authors began by observing that the:
“Use of narcotics to support patients about to undergo anesthesia has a very long history; nevertheless there are adequate reasons for examining this practice objectively. Such an examination begins logically with questions as to the purposes of the practice, its undesirable effects, and whether or not there are better ways to attain the desired results.”
Cohen and Beecher observed that in the 1930s, some practitioners found that morphine decreased the quantity of anesthetic needed to achieve a desired level of cyclopropane and ether anesthesia [29,30], while others reported that morphine contributed little, or not at all, to a reduction of the amount of ether required for a given plane of anesthesia [31,32]. Given the ubiquitous use of morphine premedication (in 1951), Cohen and Beecher asked whether the basis for such use was valid. Accordingly, they studied 558 hospitalized patients undergoing surgery [28]. Three premedication solutions were given intramuscularly (one injection per patient): 15 mg morphine and 0.6 mg atropine, 90 mg pentobarbital and 0.6 mg atropine, or 0.6 mg atropine. The contents of the solutions were unknown to the floor nurse, patient and anesthesiologist. A preoperative interview was carried out in the operating room and the anesthesiologist recorded his/her view of whether morphine had been used in the premedication.
From the anesthesiologist’s perspective the characteristics of induction were similar in all three groups and there was equal satisfaction with all the premedication solutions including atropine alone [28]. Furthermore, 47% of the time the anesthesiologist could not determine if morphine had been administered and there was an equal tendency to confuse the presence of pentobarbital with that of morphine. Moreover, the anesthesiologist mistakenly thought 34% of the atropine group had received morphine. The premedication did not affect venous blood concentrations of ether or cyclopropane during surgical anesthesia (i.e., did not appear to decrease anesthetic requirements).
Cohen and Beecher concluded that pentobarbital premedication was an effective and satisfactory substitute for morphine and was viewed as having fewer hazards. They further observed that their results should not imply that morphine was to be discarded entirely, but rather its use should be restricted to the few patients coming to surgery who were in pain.
This author (RKS) wonders if Cohen and Beecher would have reached the same conclusions if scopolamine rather than atropine had been administered with morphine in their study patients? When a calm and sedated patient is desirable, as before major and sometimes life-threatening surgery, the combination of scopolamine with morphine is highly effective and predictable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







