Fig. 58.1
British nurse Florence Nightingale during the Crimean War in the 1850s, is credited by many with having established the first intensive care unit. (Florence Nightingale (1820–1910) in the Military Hospital at Scutari during the Crimean War, 1856 (color litho) by Joseph-Austin Benwell (fl.1865–86). National Army Museum, London/The Bridgeman Art Library. Nationality/copyright status: English/out of copyright.)
Some authors refer to an earlier postoperative ward in the Newcastle Infirmary, England, around 1801, with “two five-bed rooms adjacent to the operating theatre”, reserved for the sickest patients and those recovering from surgery [2]. If the latter is correct, then the first efforts in high-dependency care preceded both Nightingale’s efforts and the introduction of general anesthesia by several decades. Recovery rooms for patients undergoing ether anesthesia were introduced in the 1870s, in the Massachusetts General Hospital in the US. These facilities probably provided immediate care after anesthesia, and thus cannot be regarded as predecessors of ICUs. Postoperative high-dependency care units appeared on both sides of the Atlantic in the 1920s in parallel with the development of more complex surgical procedures.
Early Steps in Technological (R)evolution
In addition to the concentration of human resources, the application of technology and use of invasive therapeutic interventions have been associated with ICM. General anesthesia, airway management, mechanical ventilation and vascular access in the nineteenth and early twentieth centuries developed in parallel, and independently rather than evolving in association with high-dependency care concepts. Nevertheless, these parallel developments laid the foundation for ICM as we know it today. Ake Grenvik, one of the pioneers of critical care medicine in the US, and Michael Pinsky recently reviewed the evolution of the ICU as a clinical center, and ICM as a discipline, using the development of technology as the main reference [3]. According to Grenvik, “intensive care medicine owes its roots to the support of failing ventilation.”
If so, perhaps it began with Vesalius, who described the use of positive pressure ventilation in the sixteenth century (a partial translation of the original reference “De Humani Corporis Fabrica” by Vesalius is available on the Internet at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1965933/pdf/bullnyacadmed00859-0008.pdf):
“That life may in a manner of speaking be restored to the animal, an opening must be attempted in the trunk of the trachea, into which a tube of reed or cane should be put; you will then blow into this, so that the lung may rise again and the animal take in air. Indeed with a slight breath in the case of the living animal, the lung will swell to the full extent of the thoracic cavity, and the heart will become strong and exhibit a wondrous variety of motions.”
It was then forgotten for centuries.
New trends and techniques in thoracic surgery in the early twentieth century triggered the next major steps in technology related to sustained artificial ventilation. Ferdinand Sauerbruch (1875–1951)’probably as notorious as he was famous for his surgical skills’influenced the evolution of future intensive care technologies [4] (Fig. 58.2). In 1904 he used a subatmospheric surgical chamber to inflate the lungs of patients undergoing thoracic surgery, but he forbade the addition of positive pressure ventilation. The patient’s head was outside the chamber, with the neck tightly sealed, and the patient’s body and the surgical team were inside the chamber. This created conditions equivalent to continuous positive airway pressure (CPAP), maintaining lung volume and supporting oxygenation by preventing atelectasis and thereby shunting, while the chest was open. Although the lungs might be inflated, diaphragmatic movement produced minimal ventilation when the chest was opened, and supplementary oxygen was necessary to prevent cyanosis’indicating progressive hypoventilation and increased alveolar pCO2. Sauerbruch thus advanced the development of future intensive care technology by demonstrating some of the beneficial effects of CPAP. At the same time, he may also have delayed other developments, most notably the use of tracheal intubation, positive pressure ventilation, and, as we will see later, vascular access. The sub-atmospheric surgical chamber shows Sauerbruch as the brilliant surgeon and innovator; the hindering of tracheal intubation and vascular access exemplify Sauerbruch’s negative influence.

Fig. 58.2
In the early 1900s, German surgeon Ferdinand Sauerbruch invented a subatmospheric surgical chamber to prevent lung collapse in patients undergoing thoracic surgery, thereby demonstrating the benefits of continuous positive airway pressure. [Photo originally published as Plate 5 inThe Dismissal: the Last Days of Ferdinand Sauerbruch, Surgeon, by Jurgen Thorwald. Thames & Hudson: London, 1961. Original copy owned by the National Library of Australia. Instructions also say to include persistent identifier or call number, possibly 610.92 SAU (2555658)]
Franz Kuhn (1866–1929), a German surgeon, described orotracheal intubation in 1902–1911 [5]. Others proposing tracheal intubation at the end of the nineteenth century included Karl Maydl (1853–1903), a Prague surgeon, and the Viennese physician Viktor Eisenmenger (1864–1932), but their ideas did not receive the attention that they deserved. Sauerbruch vigorously opposed the use of orotracheal intubation, and effectively delayed its introduction to clinical practice. Orotracheal intubation came into wide-spread use in the 1940s after the introduction of curare into anesthesia.
In the late 1920s, the first device suitable for prolonged artificial ventilation, the iron lung, was invented. Unlike the inventors of the surgical negative pressure chamber, the inventors of the iron lung’Philip Drinker and Louis Shaw’were interested in more than a short-term solution: their 1929 paper was appropriately titled “An apparatus for the prolonged administration of artificial respiration” [6]. John Emerson, the founder of the JH Emerson Co further developed their invention for larger-scale commercial production [7]. The iron lung (or tank respirator) enclosed the patient within a metal cylinder up to the head and neck, with an airtight collar surrounding the neck. Cyclic intermittent negative pressure around the body within the cylinder effected ventilation. Major problems in long-term use related to reliability, respiratory tract care, accessing the abdomen and thorax and comfort. Cuirass negative pressure ventilators were a modification of the iron lung, restricted to enclosing only the thorax. Although the cuirass allowed better access and was far less expensive, it was less effective as a ventilator, and nursing care and comfort remained problematic. Neither device solved the crucial problem of the patient who could not maintain their own airway.
Major developments in vascular access paralleled the early steps of ventilatory support. The young German surgeon-in-training Werner Forssmann (1904–1979) summarized the problems related to the lack of central vascular access in the introduction of his 1929 paper “Die Sondierung des rechten Herzens” [8] (Right heart catheterization; freely translated from German by me): “In acute dangerous situations, where the patient is at risk of cardiac arrest, i.e. by acute collapse, in cardiac failure, or through complications of anesthesia or poisoning, rapid treatment with drugs locally is necessary. In such cases, attempting an intracardiac injection is often the only possibility to save the patient.” Forssmann then described the complications of cardiac puncture and proposed right heart catheterization as the solution [9]. He tested the feasibility of such a procedure during autopsies. Then, with the help of a “friendly colleague”, Forssmann advanced a well-lubricated ureteral catheter via his right brachial vein to a depth of 35 cm and verified the intrathoracic position of the catheter using X-ray. At this stage he broke off the experiment at the urging of the colleague, who considered the procedure too risky. One week later, Forssmann advanced a 65-cm-long ureteric catheter through his own left brachial vein to its full length, verifying the catheter tip in the right atrium by chest X-ray’he was disappointed that he did not choose a longer catheter. He then described the first clinical use in a patient in severe septic shock who initially responded favorably to fluids and drugs but died after a few hours. Sauerbruch rewarded Forssmann by firing him. Undeterred, Forssmann continued to develop right heart catheterization and pulmonary angiography as a diagnostic tool.
According to Forssmann [9], O Klein made the first measurements of cardiac output using a right heart catheter and the Fick principle in 1930 [10]. Next, Andre Cournand advanced his special catheters into the pulmonary circulation in the late 1930s and early 1940s, and shared the Nobel Prize in physiology/medicine with Forssmann in 1956 [11]. It took more than 40 years after Forssmann’s initial experiments to convert these tools to practical monitoring devices.
Polio Epidemics Jump Start Modern ICM
An outbreak of polio in California in the late 1940s resulted in many patients needing tank and cuirass ventilators and intermittent positive pressure ventilation. There is inconsistency in the reported numbers of patients that were treated with the different methods. Albert Bower and Ray Bennett reported their experiences with ventilator support in nearly 300 cases in 1948 [12].
The Californian epidemic was vastly overshadowed by the catastrophic polio epidemics in the early 1950s prompting the next important technological and clinical developments in prolonged mechanical ventilation. The response to the epidemics, particularly the 1952 epidemic in Copenhagen, led to the development of ICM.
Numerous authors have debated details surrounding the Copenhagen polio epidemics and the roles of the various players [13–15]. The doctors, nurses, and other personnel confronted with the initial high mortality, and the large number of new patients arriving daily, had priorities that precluded precise record keeping. It is also meaningless to debate the “firsts”. The heroic efforts of individuals worldwide, especially Bjørn Ibsen, the pioneering intensivist [13–16], have been described in detail, and are also surrounded by folklore. I will summarize the framework, give an approximate temporal sequence of events, and then try to explain why the Copenhagen polio epidemic was so special and why it had such a large impact on the evolution of ICM.
As detailed elsewhere in this book, the late 1940s was an era of rapid development in anesthesiology. Departments of Anesthesia had been established in major hospitals, and the first positive pressure ventilators had been introduced for perioperative use during thoracic surgery. The role of anesthetists in Europe was undergoing a major change. European anesthetists visited the US, going especially to the Massachusetts General Hospital for training. Concurrently, various anesthesia-related activities commenced in Europe’particularly in Sweden. A commission for future organization of anesthetic services in Copenhagen recommended the establishment of independent departments of anesthesia for each hospital. The World Health Organization founded the Anaesthesiology Centre Copenhagen, in May 1950, drawing leading anesthetists from the UK, Sweden and the USA to teach in a one-year anesthesia training course. The “ primus motor” (“prime mover”) of this course was Erik Husfeld, a thoracic surgeon, who served as the course director from the first course until 1973. Thus, perhaps fortuitously, there was already a considerable focus in Copenhagen, on this “specialty in the making [14].”
The scale of the epidemic, and the ultimate success in saving lives in Copenhagen, received much publicity. The struggle with polio elsewhere is less well known. During the polio epidemic in many other countries, medical communities endured frustrating experiences treating the severely paralyzed patients. In postwar Europe, resources were scarce, and innovative solutions were badly needed. In Germany, Alfred Dönhart, a pulmonologist from Hamburg, together with the Dräger company, converted old submarine torpedo-tubes into iron lungs (Fig. 58.3). Dräger became a major producer of ventilators, anesthesia machines, and other medical equipment.
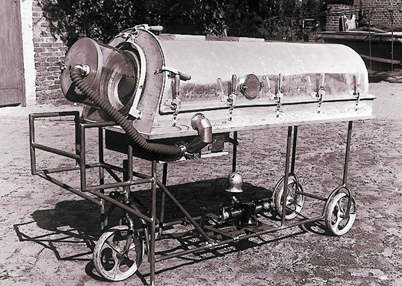
Fig. 58.3
A prototype of an “iron lung”, developed by the German firm Dräger in 1947. (Courtesy of Drägerwerk AG & Co. KgaA.)
In California, clinician Bower and engineer Ray Bennett described a comprehensive approach to the patient with respiratory failure, in their 1950 [9] paper reporting the results of their treatment of more than 70 polio patients with their combined approach, reporting improved outcomes with positive pressure ventilation in comparison with the use of the tank ventilator alone. They developed different types of positive pressure devices, primarily for use in combination with the tank ventilator, but also capable of supporting ventilation via mask during nursing care. Why they insisted on the combined use of the tank ventilator and the positive pressure device is unclear. They also developed tracheostomy tubes specifically for prolonged assisted ventilation. Blood gas analyses were used to assess the adequacy of ventilation. Humidifiers, monitoring devices and alarm systems were added. Special mechanical mattresses were developed to prevent pressure sores.
Until recently, these accomplishments received surprisingly little attention, although Ibsen, the pioneer in the subsequent Copenhagen epidemics, gave Bower and Bennett the credit they deserved. Bennett established a company manufacturing positive pressure ventilators, and the successor of his original company (Puritan-Bennett), became a market leader in intensive care ventilators worldwide.
The Danish polio epidemic began in August 1952, and by the end in in April 1953, almost 5700 polio cases had been registered throughout the country. In Copenhagen, Blegdam Hospital received many of the more seriously ill victims, including 345 patients with either breathing or swallowing difficulties or both. During the second half of August 1952, the number of cases, especially those with paralysis, increased at an alarming rate. Eighty-seven percent of patients with bulbar polio (27 of 31) died. Most physicians incorrectly thought that these patients died from irreversible cerebral lesions of polio. Tank or cuirass negative pressure ventilators were used to support ventilation in patients with respiratory paralysis, but only one tank and 6 cuirass respirators were available.
The high mortality rate and rapid increase in new cases persuaded the chief of the hospital and professor of epidemiology, HC Lassen, to consult with Bjørn Ibsen’an anesthetist. Aware of the reports of Brower and Bennett from the California polio outbreak, Ibsen became convinced that the patients suffered from progressive severe hypercapnia, not irreversible brain injury. He believed that the high blood CO2 content resulted from hypoventilation and not metabolic alkalosis, as was thought at the time. Ibsen convinced his skeptical colleagues to undertake a trial in a test patient, with tracheostomy and manual positive pressure ventilation. After initial difficulties (it was initially impossible to inflate the patient’s lungs due to secretions and bronchospasm, followed by hypovolemic shock), the patient’s condition was stabilized. Temporarily transferring the patient back to the cuirass resulted in cyanosis, which disappeared on returning to positive pressure ventilation. This convinced Lassen and his colleagues that Ibsen was correct. The new strategy was then applied to all polio patients with respiratory failure. This was no simple task, since at the peak of the epidemic, 30 to 50 new patients per day were admitted to Blegdams, 6 to 12 with breathing or swallowing problems or both. 1500 students were enlisted over the course of the epidemic to manually ventilate the patient’s lungs around the clock. [17] The new strategy decreased mortality from 90 to 25% in those severe cases. The personal memoir of one of the student “ventilators” gives special insight into the circumstances in Copenhagen [17].
I believe that the success of Ibsen, Lassen, and co-workers illustrates the approaches needed to solve problems in critically ill patients. First, Ibsen used clinical observations based on sound physiologic concepts. Second, he introduced a novel application of existing methodology. Third, he developed a treatment strategy. Fourth, he convinced the decision makers to supply substantial resources. Fifth, a multidisciplinary team was used. Sixth, the logistics necessary for the prolonged treatment of large numbers of patients were established. Seventh, the outcomes of the new strategy were carefully assessed.
The use of positive pressure ventilator support for polio patients with respiratory failure spread rapidly in Scandinavia, continental Europe, and the United Kingdom. It stimulated the development of positive pressure ventilators. The prototype of the Engström ventilator had already been tested in Copenhagen, and was further developed by Carl-Gunnar Engström before the 1953 Swedish polio epidemic. [15] Engström’s company became a major ventilator manufacturer, and was subsequently acquired by Datex’the Finnish manufacturer of anesthesia and intensive care technology and later by General Electric.
The need to understand the pathophysiology of respiratory failure, and monitor its treatment, accelerated the development of blood gas analysis technology. Analysis of blood gases in the early 1950s was applied during the Copenhagen polio epidemic, but required a glass electrode to measure blood pH, a Van Slyke apparatus to measure CO2 content, and application of the Henderson-Hasselbalch equation to calculate pCO2. To circumvent this cumbersome and labor-intensive analysis, Poul Astrup introduced a new approach based on the linear relationship between blood pH and the logarithm of the pCO2 [18]. This was subsequently used by the Radiometer company’a leading producer of blood gas analyzers today. A more detailed description of this development appears in Chapter 41.
ICUs Arise in the Post-polio Era
Modern ICUs arose in the wake of the polio epidemic. It was not a “big bang” but rather an evolution in which the concepts of managing respiratory failure from polio were applied to a wider patient population: providing intermittent positive pressure ventilation with mechanical ventilators, concentrating human resources, and congregating patients requiring intensive care, thus enabling continuous treatment and monitoring. Among many “firsts”, it appears that the first ICU started at Kommunehospitalet, the Municipal Hospital of Copenhagen, in December 1953, under Ibsen’s leadership.
In the next decade, ICUs developed in Europe, the US, and Australasia. The new possibilities for prolonged mechanical support of breathing made mechanical ventilation and management of respiratory problems a hallmark of ICUs. In parallel, it became apparent that concentrating human and technological resources covering a range of special skills enhanced care for severely ill patients.
This evolution is evident in the National Academy of Sciences’National Research Council Committee on Anesthesia’s “Workshop on Intensive Care Units”, held 14 October 1963 [19]. The pioneers attending the meeting pointed out problems related to intensive care that remain challenging today: the role of different specialties in the treatment of patients with multidisciplinary problems; the need for special training of nurses and doctors; and the need for attending specialists to be directly responsible for patient care.
John Kinney, a future pioneer in metabolic research in the critically ill [20] at Columbia Presbyterian Medical Center, New York, pointed out that no single physician can attend to a critically ill patient 24 hours a day for 5–6 days. Yet, this was an average length of stay in many units then, and now, necessitating shared responsibility’a fact still denied in many hospitals today. Henning Pontoppidan from the Massachusetts General Hospital, also best known for his contributions to management of acute respiratory failure [21], presented one of the first patient classification systems relevant for ICM. He defined “critical care” as care of patients actually or potentially needing life-saving devices or activities, and needing highly skilled nursing care and close and frequent, if not constant, nursing observation. Peter Safar, who developed ICM in Baltimore and Pittsburgh, summarized the prerequisites for smooth functioning of intensive care: interdepartmental cooperation, well-defined responsibilities and authority, efficient design of ICU facilities, and standardization of certain procedures’principles that are all applicable today.
The topics discussed at the 1963 “Workshop on Intensive Care Units” reflected important international developments in intensive care. An influx of European anesthesiologists facilitated the adoption of the innovations of Ibsen and his colleagues in the US, after an initial delay, European anesthetists had already established connections in the US in the late 1940s, through training in anesthesiology, particularly at the Massachusetts General Hospital (MGH). Henrik Bendixen came to Boston to start his anesthesia residency in 1954, having graduated in Copenhagen in 1951. Henning Pontoppidan graduated one year later and also came to the MGH. Myron Laver and John Hedley-White shared Bendixen’s and Pontopiddan’s interests in respiratory and cardiovascular pathophysiology. This group of innovators produced physiologic and clinical research that established the basic tenets underlying mechanical ventilation in the operating room and in ICM [22–23]. They helped found the first respiratory ICU at the MGH in 1966.
Until the mid-1960s, mechanical ventilation with continuous positive pressure was avoided, largely because Cournand’s early work in the late 1940s indicated that continuous positive pressure decreased cardiac output. But then David Ashbaugh and Thomas Petty published “Acute Respiratory Distress in Adults” (ARDS) in theLancet in 1967 [24], reportedly after rejection by three major American journals. They described the use of positive end-expiratory pressure (PEEP) to correct severe hypoxemia during mechanical ventilation, in 12 patients with hypoxemia refractory to mechanical ventilation without PEEP. This led several groups of researchers, including the Boston group, to study the underlying mechanisms. By the early 1970s Pontopiddan, Laver, and co-workers established that the functional residual capacity of the lungs in ARDS was dramatically reduced, and that it could, at least in part, be restored by applying continuous positive pressure [25]. More Europeans came to Boston, among them Konrad Falke from Germany, who after participating in some of the important studies on PEEP [26], became a leader in using extracorporeal lung support in Europe, and a long-term Editor-in-Chief ofIntensive Care Medicine.
ICM Evolution in Europe
Europeans who went to the US defined much of the evolution of ICM in North America, and ICM in Europe profited from knowledge contributed by intensivists returning from the US. Still, the 1950s to the 1970s produced less interaction between English-speaking and non-English-speaking medical communities than today’partly due to language and cultural barriers.
In Germany, the first ICUs appeared in the late 1950s [27], well after 1953 reports of a large case series of patients requiring prolonged ventilation using the iron lung. Mechanical ventilation was an important theme in France as well’however, the French focused early on infections, with the rest of the world following suit decades later. Creative individuals in France had a decisive impact. One such person was Maurice Rapin in Paris who along with his colleagues applied mechanical ventilation to a broad spectrum of patients. His first publications on the use of prolonged mechanical ventilation stem from 1955, in patients with tetanus [28]. They were followed by applications in post-pneumonectomy respiratory failure [29], various paralytic conditions including polio, and postoperative respiratory failure of various etiologies. In 1958, he published two papers outlining the problems of respiratory failure in patients with chronic obstructive pulmonary disease (COPD) [30,31]. Published in French, neither paper received the attention it deserved. Rapin then published several papers on barbiturate intoxication, and in 1960, his first paper on septic shock. In 1963, he described the risks of nosocomial infections (“Infectious complications in respiratory resuscitation. Etiological, symptomatic and therapeutic data”) [32].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







