CHAPTER 85 THE DIAGNOSIS AND MANAGEMENT OF CARDIAC DYSRHYTHMIAS
With increasing frequency, patients undergoing surgery have multiple medical comorbidities, not necessarily associated with their surgical disease. Cardiac dysrhythmia in this setting can be a primary event, a secondary event related to ischemia or myocardial infarction, or may be due to toxic or metabolic abnormalities associated with perioperative care. The type of dysrhythmia can usually be diagnosed with a focused physical exam, a standard 12-lead electrocardiogram (ECG), and from the response to specific maneuvers or drug therapy. The acute management depends on the hemodynamic stability of the patient, the accurate classification of dysrhythmia, and an understanding of the underlying mechanism so that appropriate treatment can be given in an expeditious fashion. Management may include a combination of cardioversion for the acutely unstable, pharmacological intervention, percutaneous or transvenous pacing, or modalities such as aberrant pathway ablation and implantation of pacemakers or defibrillators.
INCIDENCE
The incidence of dysrhythmia following cardiac surgery can be high as 40% in some studies, with a large proportion caused by atrial fibrillation. It is well known that up to 15% of patients with inferior wall myocardial infarction present with atrioventricular (AV) nodal conduction disturbances or complete heart block. Patients with pre-existing cardiac or pulmonary disease have an increased risk of dysrhythmia, which is compounded in the face of noncardiac surgery, trauma, or other critical illness. Vasopressor requirement is associated with an increased risk of dysrhythmia due to the proarrhythmic effect of catecholamines on the heart. It has been shown that dysrhythmias, in a standard intensive care unit (ICU) that admits cardiac, noncardiac, and medical critical care patients, occur in up to 20% of patients. The vast majority are tachyarrhythmias, while 10% are bradyarrhythmias. Dysrhythmias are distributed equally between atrial and ventricular origin. Atrial fibrillation and ventricular tachycardia are by far the most common. Patients that have dysrhythmias have longer ICU stays and had worse survival overall, likely signifying that dysrhythmias in patients are an indicator of more severe critical illness.
BRADYARRYTHMIAS
Atrioventricular Node
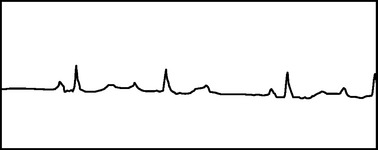
Disturbances in conduction through the AV node or His-Purkinje system are classified as atrioventricular blocks. These may be temporary or permanent, depending on the etiology of the delayed conduction. In adults, the most common causes are drug toxicity, coronary artery disease, and degenerative disease of the conduction system. Many other conditions, such as electrolyte disturbances, myocarditis, sarcoidosis, scleroderma, and hypervagal responses, can cause AV block. The P-R interval is a measure of the conduction time through the AV node and bundle of His. When the P-R interval is prolonged (>210 milliseconds), a patient has first-degree AV block. Second-degree AV block is when intermittent failure of the conduction of the impulse to the ventricles occurs. In Mobitz type I, second-degree AV block (Wenckebach block), there is progressive prolongation of the P-R interval until failure of conduction to the ventricle occurs (Figure 1). The P-R interval then shortens following the dropped beat. This failure in conduction originates from the AV node itself and the QRS complex remains narrow. In Mobitz type II, second-degree AV block, there is intermittent failure of conduction reaching the ventricles that is not associated with progressive prolongation of the P-R interval. There is not a shortened P-R interval following the dropped beat. This failure in conduction is considered “infranodal” and originates from the His-Purkinje system. The QRS complex may be prolonged, and this type of AV block is more concerning. There is a significant likelihood of progression to complete heart block associated with inadequate ventricular response with this rhythm. Third-degree or complete heart block results from failure of all impulses through the AV node and His-Purkinje system, resulting in atrioventricular disassociation. The ventricles rely on their innate automaticity which produces a typical wide QRS escape rhythm between 40 and 50 beats per minute. The atrial rate is commonly faster, producing multiple P waves with no relationship to the ventricular QRS complexes (Figure 2).
Management of AV block depends on the hemodynamic stability of the patient, the transient nature of the dysrhythmia, and where the focus originates from within the conduction system. Acute pharmacotherapy relies upon atropine and isoproterenol. Isoproterenol use should be avoided in patients with ischemia heart disease because of the associated increase in myocardial oxygen demand. There is no long-term pharmacotherapy for AV block, and removal of any of the common offending agents, such as digitalis or beta-blockers, should first be attempted. Temporary pacing is used for those with ongoing instability, and permanent pacing is typically required for Mobitz type II second-degree AV block and third-degree AV block.
TACHYARRHYTHMIAS
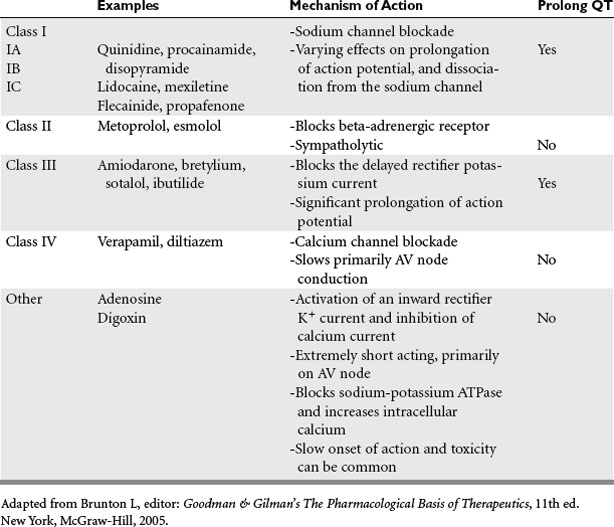
Tachyarrhythmias are classified according to their anatomical origin in relation to the AV node. Those that originate at or above the AV node are considered supraventricular tachyarrhythmias; the most relevant include sinus tachycardia, paroxysmal supraventricular tachycardia, multifocal atrial tachycardia, atrial flutter, and atrial fibrillation. Ventricular tachyarrhythmias originate from below the AV node and include ventricular tachycardia and ventricular fibrillation. Important determinants of the malignant potential of these tachyarrhythmias are the duration, the hemodynamic consequences, and the presence of significant structural heart disease. The acute management depends on a basic understanding of the mechanism, the choices for pharmacological intervention (Table 1), and the indications for urgent cardioversion for each situation. Interventional techniques, such as aberrant pathway ablation and implantation of pacemakers and defibrillators, have drastically improved long-term outcome once patients have left the ICU setting, and have added significantly to our armamentarium in treating these dysrhythmias.
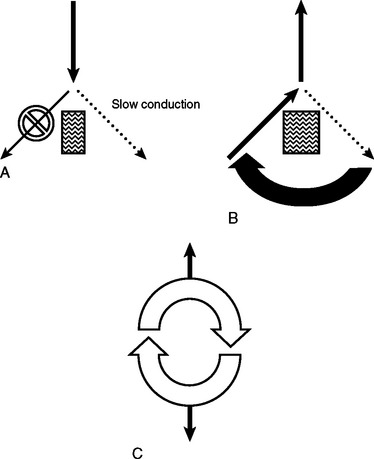
The mechanism by which tachyarrhythmias arise are categorized into (1) abnormal automaticity, (2) triggered activity, or (3) re-entry. Abnormal automaticity occurs when cells outside the normal conduction system generate spontaneous impulse formation. Triggered activity occurs during “after depolarizations,” which cause the membrane potential to reach threshold early and generate abnormal impulse formation. Re-entry, the most common mechanism, occurs when an impulse can travel down two pathways separated by an area of unexcitable tissue. One of the pathways contains a unidirectional block, with slowed conduction, so that recovery and further excitation can subsequently occur. This defines an area of cardiac tissue that can self-propagate and thus becomes the focus for the generation of the tachyarrhythmia (Figure 3).
Sinus Tachycardia
Sinus tachycardia should be considered a physiological reflex rather than a true dysrhythmia, but it is an important sign for which the etiology must be sought. Fever, hypovolemia, and anemia all appropriately increase heart rate to at least maintain or increase cardiac output. Blunting this reflex with pharmacotherapy without knowledge of the etiology can be dangerous. Thyrotoxicosis, pheochromocytomas, or side effects of sympathomimetic drugs also cause sinus tachycardia without regard for reflex mechanisms. The primary goal for management of sinus tachycardia is to find and appropriately treat the confounding condition. Sinus tachycardia will resolve with resolution of this inciting process.
Paroxysmal Supraventricular Tachycardia
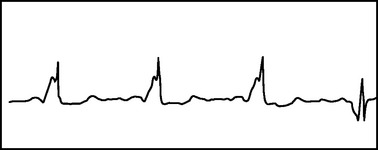
When the accessory pathway has the potential for antegrade conduction, the QRS complex will be wide, since conduction occurs between the ventricular myocytes themselves rather than the His-Purkinje system. Wolfe-Parkinson-White syndrome occurs when the accessory pathway allows both antegrade and retrograde conduction, and commonly a delta wave or pre-excitation can be seen in the early QRS complex (Figure 4). This syndrome can present in early adulthood, and the initial presentation can be ventricular fibrillation. Management in these patients where conduction occurs antegrade through the accessory pathway varies from narrow complex AVRT as described previously. Adenosine will only be effective if the antegrade conduction occurs through the AV node. Otherwise it can precipitate atrial fibrillation, which can result in degeneration to ventricular fibrillation when an antegrade accessory pathway exists in these patients. Calcium channel blockers and digoxin are contraindicated, because they will slow conduction in the AV node and enhance conduction through the accessory pathway. Class I and class III antiarrhythmics, which include flecainide, procainamide, and ibutilide, are able to depress the conduction across the accessory pathway, decrease the ventricular rate, and likely terminate the wide QRS tachyarrythmia. Direct-current cardioversion is used with hemodynamic instability or if failure of antiarrhythmic therapy occurs.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree