Agent (drug)
Availability
Cost
Purity/potency
Mode of administration
Chewing (absorption via oral mucous membranes)
Gastrointestinal
Intranasal
Subcutaneous and intramuscular
Intravenous
Inhalation
Speed of onset and termination of effects (pharmacokinetics: combination of agent and host)
Host (user)
Heredity
Innate tolerance
Speed of developing acquired tolerance
Likelihood of experiencing intoxication as pleasure
Metabolism of the drug (nicotine and alcohol data already available)
Psychiatric symptoms
Prior experiences/expectations
Propensity for risk-taking behavior
Environment
Social setting
Community attitudes
Peer influence, role models
Availability of other reinforcers (sources of pleasure or recreation)
Employment or educational opportunities
Conditioned stimuli: environmental cues become associated with drugs after repeated use in the same environment
Furthermore, abuse of tobacco, alcohol, and illicit drugs is costly. In the USA it is more than $700 billion annually spent in costs related to crime, lost work productivity, and healthcare [2].
Addiction was historically viewed as a disease of “weak personality” and was not systematically addressed by the scientific and medical communities until the latter half of the twentieth century. Addictions are now commonly accepted as diseases of the brain caused by the impact of the drug itself on the brain and modified by various environmental factors (see Table 21.1). Further, the presence of specific variants of multiple genes may enhance or decrease the vulnerability to developing specific addictions [3].
Epidemiology provides the foundation for understanding drug use, abuse, and dependence by demonstrating the distribution and determinants of these disorders. Recent findings from several studies of nationally representative samples in the USA reveal that the lifetime prevalence of alcohol use disorders is approximately 8%, and illicit drug use disorders is 2–3% [4].
Furthermore, in 2014, an estimated 27.0 million Americans aged 12 or older were current (past month) illicit drug users. The most commonly used illicit drug in the past month was marijuana, which was used by 22.2 million people aged 12 or older. An estimated 6.5 million people reported nonmedical use of psychotherapeutic drugs in the past month, including 4.3 million nonmedical users of prescription pain relievers (Fig. 21.1).
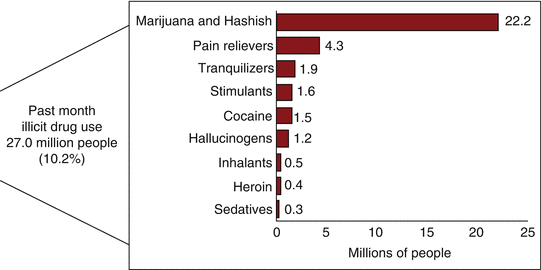

Fig. 21.1
Numbers of past month illicit drug users among people aged 12 or older in 2014 in the USA. From Center for Behavioral Health Statistics and Quality [47], with permission
These addicted patients may present for anesthetic care in a variety of circumstances: in obstetrics for labor or cesarian sections, in trauma for emergency surgeries, in lifesaving situations (resuscitation), and in everyday elective surgeries. Therefore, it is important for anesthesiologists to know about the most common illicit drugs being used, their clinical presentation and side effects, and to know what anesthetic options would be beneficial or detrimental [5].
In this chapter, we present some of the most frequently used illicit substances and their effects and importance for anesthesiologists. We also discuss the management of addicted patients prior to administering anesthesia or analgesia, allowing us to predict adverse drug interactions, predict tolerance to some anesthetic agents, and recognize drug withdrawal signs and symptoms.
21.1.1 Neurobiology of the Addicted Patient
All drugs of abuse affect the brain’s “reward circuit,” with the mesolimbic dopamine pathway being of particular importance. This pathway includes dopaminergic neurons extending from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) [6]. Dopamine levels that are either too high or too low are suboptimal and may lead to impulsive and risk-taking acts including excessive substance use. Natural rewards and abused substances appear to induce similar activity in reward circuitry and connected regions, including the amygdala, hippocampus, and frontal cortex [6]. Furthermore, the crucial role of other neurotransmitters (e.g., glutamate, GABA, cannabinoids, opioids, serotonin) in reward processing and in the neuroadaptations associated with addiction has recently been appreciated [7]. It is now recognized that drugs of abuse disrupt (either increasing or decreasing) the strength of excitatory synapses by tapping into traditional mechanisms of plasticity, including long-term potentiation (LTP) and long-term depression (LTD) [7].
Synaptic plasticity is controlled presynaptically through the regulation of glutamate release and postsynaptically through the insertion or removal of AMPA or NMDA glutamate receptors, and drugs of abuse interfere with these processes [8]. The shift from normal healthy desire to drug craving with increased levels of drug use is also associated with changes in limbic, striatal, and cortical brain systems [9]. Mesocorticolimbic dopamine pathways, which arise from the midbrain VTA, have a critical role in the mediation of reward. In particular, the VTA dopamine projection to the NAc (part of the ventral striatum) has a prominent role in positive reinforcement (Fig. 21.2). In brief, amygdala circuits contribute to the formation of associative reward- and fear-related memories, hippocampal circuits are critical for declarative memory functions, and frontal cortical circuits mediate control of executive functions. In turn, innervation of the NAc by each of these circuits allows sensory and emotional information to be converted into motivational actions through the output to extrapyramidal motor systems. Dopamine signaling in the dorsal striatum has a key role in the development of compulsive forms of reward seeking and consumption [10].
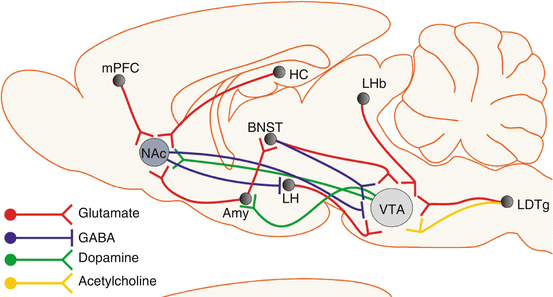

Fig. 21.2
Brain regions of major importance for the acute and chronic addictive effects of drugs of abuse. Reproduced from Parsons and Hurd [10], with permission. The drawing shows many pathways that participate in rewarding/drug-seeking behavior. Amy amygdala, BNST bed nucleus of stria terminalis, HC hippocampus, LDTg laterodorsal tegmental nucleus, LH lateral hypothalamus, LHb lateral habenula, mPFC medial prefrontal cortex, NAc nucleus accumbens, VTA ventral tegmental area
Natural rewards, such as food, sex, and exercise, and drugs of abuse—including psychostimulants (such as cocaine and amphetamine), nicotine, alcohol, opioids, and cannabinoids—increase NAc dopamine levels, and this neurochemical response contributes to subjective reward and positive reinforcement [11]. Furthermore, certain mechanisms seem to be common for many drugs, for example, activation of the extracellular signal-regulated kinase (ERK) pathway in specific brain structures is necessary for effects of and tolerance to cocaine, nicotine, MDMA, phencyclidine, alcohol, and cannabinoids after both acute and chronic treatments in rodents [10].
Finally, genes account for about 50% of a person’s risk of becoming addicted, and environmental factors influence the effect of these genes—an area of research called epigenetics. Progress in genetics/epigenetics research will lead to more refined prevention and treatment interventions targeted to individual risk or to modifiable environmental influences [2].
21.1.2 Psychological Aspects of the Addicted Patient
Like most other mental health problems, drug use disorders have no single cause. However, there are a number of biological, psychological, and social factors, known as risk factors, that can increase an individual’s vulnerability to developing a chemical use disorder. Psychologically, intoxication with or withdrawal from a substance can cause everything from euphoria as with alcohol, ecstasy, or inhalant intoxication, to paranoia with marijuana or steroid intoxication, to severe depression or suicidal thoughts with cocaine or amphetamine withdrawal [12]. Epidemiological studies found the prevalence of substance use disorder, a common key feature of several serious psychiatric illnesses such as bipolar disorder, schizophrenia, major depressive disorder, and attention deficit hyperactivity disorder, to be high compared to the general population [13]. Furthermore, it seems that psychiatric diseases make the brain more susceptible to addiction. This observation is supported by studies in animal models suggesting that psychiatric disorders potentiate behavioral and reinforcing effects of drug of abuse [13].
There are many forms of evidence-based behavioral treatments for substance abuse. Some of the most strongly supported include cognitive behavioral therapy (CBT) that can help addicted patients overcome substance abuse by teaching them to recognize and avoid destructive thoughts and behaviors, motivational interviewing that involves structured conversations that help patients increase their motivation to overcome substance abuse, and contingency management that provides tangible incentives to encourage patients to stay off drugs [14]. These behavioral treatments can sometimes be particularly effective when combined with pharmaceutical treatments that either mimic the effects of the drug in a controlled way (such as methadone and buprenorphine for opioid addiction or nicotine chewing gum for cigarette addiction) or reduce or eliminate the “high” the user gets from the drug (such as naltrexone for opioid or alcohol addiction) [14].
21.2 Presentation of the Drugs
As already stated in Sect. 21.1, it is important for anesthesiologists to know about the most common illicit drugs being used, their clinical presentation, and side effects. This information is presented in this section.
21.2.1 Alcohol
Ethyl alcohol, or ethanol, is an intoxicating ingredient found in beer, wine, and liquor. Alcohol is produced by the fermentation of yeast, sugars, and starches. It is a central nervous system depressant that is rapidly absorbed from the stomach and small intestine into the bloodstream. Thirty years ago, little was known about the genetic basis of alcohol dependence or the nervous system changes that occur as a result of prolonged heavy drinking. Alcohol dependence was thought to be a disease of middle age. Disulfiram (Antabuse®) was the only medication approved for treating alcohol dependence, producing acute sensitivity to alcohol. Other treatments included various behavioral approaches, mostly group counseling and referral to Alcoholics Anonymous (AA) [15].
The fifth edition, DSM-5, integrates the two DSM-IV disorders, alcohol abuse and alcohol dependence, into a single disorder called alcohol use disorder (AUD) with mild, moderate, and severe subclassifications.
Concerning the etiology of alcoholism, the disease itself is considered to be a consequence of an interactive influence of the environment and genetic factors.
Recent molecular pharmacology studies demonstrated that alcohol has only a few known primary targets: NMDA, GABAA, glycine, serotonin, and nicotinic receptors as well as L-type Ca2+ channels and G-protein-activated inwardly rectifying K+ channels. Addictive behavior toward alcohol as measured by alcohol-seeking and relapse behavior involves the activity of the mesolimbic dopaminergic system which plays a crucial role during the initiation phase of alcohol consumption. Following long-term, chronic alcohol consumption virtually all brain neurotransmission seems to be affected, making it difficult to define which of the systems contributes the most to the transition from controlled to compulsive alcohol use. However, compulsive alcohol drinking is characterized by a decrease in the function of the reward neurocircuitry and a recruitment of antireward/stress mechanisms comes into place, with a hypertrophic corticotropin-releasing factor system and a hyperfunctional glutamatergic system being the most important ones [16].
Alcohol affects every organ in the drinker’s body and can damage a developing fetus. Intoxication can impair brain function and motor skills; heavy use can increase risk of certain cancers, stroke, and liver disease. Alcoholic liver disease (ALD) is a major cause of chronic liver disease, leading to cirrhosis and liver cancer. The spectrum of ALD includes steatosis, alcoholic steatohepatitis (ASH), cirrhosis, and hepatocellular carcinoma (HCC) [17]. Alcohol metabolism involves several enzymes, including alcohol dehydrogenase, acetaldehyde dehydrogenase, and CYP2E1. Since the risk of developing ALD is mediated by genetic polymorphisms in these enzymes, an understanding of the genetic predisposition of the individual is required. Moreover, CYP2E1 is involved in the metabolism of various other drugs, potentiating drug-drug interactions. Another important aspect of ALD progression is its comorbidity with chronic viral hepatitis. The interaction between alcohol and medications used to treat HCV can exacerbate the degree of hepatotoxicity [17].
Efforts to develop medications for alcohol use disorders have expanded rapidly in recent years. In addition to disulfiram, naltrexone and acamprosate are now approved for use in treating alcohol dependence. When used in conjunction with behavioral therapies, medications improve the chance for recovery and the lives of those who suffer from alcohol dependence. Several behavioral approaches, such as motivational enhancement therapy, cognitive behavioral therapy, and twelve-step facilitation therapy, are effective in treating alcohol dependence [15].
21.2.2 Cannabis
The cannabis plant, which is also known as hemp or marijuana, is one of the oldest documented medicines in history. Cannabis contains 545 chemical compounds of which 104 are cannabinoids, the rest being flavonoids, terpenes, fatty acids, and more, all with potential medical uses. The best-characterized constituent is Δ9-tetrahydrocannabinol (Δ9-THC), the principal psychoactive component of cannabis. Other important constituents include cannabidiol (CBD) and cannabinol (CBN). The former lacks psychoactive capabilities, whereas the latter is a mildly psychoactive chemical. Cannabinoids produce their effects through the activation of two distinct G-protein-coupled receptors termed cannabinoid CB1 and CB2 receptors. The CB1 receptor is expressed, at high levels, in the central nervous system (CNS) and along pain pathways, whereas the CB2 receptor is found predominantly, although not exclusively, outside the CNS, where it is most densely expressed in peripheral tissues with immune functions. The isolation of endogenous ligands (endocannabinoids), mainly anandamide and 2-arachidonoylglycerol, completed the discoveries in the cannabinoid field [18].
In animal models, both Δ9-THC and synthetic CB1 receptor agonists enhance brain reward function, produce rewarding effects in the paradigm of conditioned place preference (CPP), and are voluntarily self-administered (intravenously and also directly into the NAc shell and posterior VTA) [19]. These effects are critically reliant on CB1 receptor signaling and are highly dose sensitive, with a rapid shift to negative reinforcing effects with increasing dose. Although enhancement of endocannabinoid (eCB) levels does not produce rewarding effects per se, eCB signaling at cannabinoid receptors participates in the mediation and modulation of both natural and drug-induced reward. Long-term drug use leads to neuroadaptive downregulation of eCB signaling resulting from diminished CB1 receptors and/or CB2 receptor function as well as possible disruptions in eCB biosynthesis and/or clearance [10].
Cannabis is the most widely used illicit drug in the world. Furthermore, approximately 100 million Americans have used illicit marijuana, with 8–10% developing cannabis dependence [20]. Marijuana has medicinal effects [21], including antiemetic properties, muscle-relaxing and anticonvulsant properties, and pain-relieving properties [22]. These medical benefits come at the cost of many side effects, especially psychoactive ones.
Tolerance to most of the effects of cannabis can develop rapidly after only a few doses but also disappears rapidly. Withdrawal symptoms consist of restlessness, irritability, mild agitation, insomnia, nausea, and cramping [1]. It affects only heavy smokers (on a daily basis) who suddenly stop taking it. No specific treatment has been proposed.
21.2.3 Cocaine
Cocaine is a powerfully addictive stimulant drug made from the leaves of the coca plant native to South America. Although healthcare providers can use it for valid medical purposes, such as local anesthesia for some surgeries, cocaine is an illegal drug. Street dealers may mix it with other drugs such as amphetamine. People snort cocaine powder through the nose or rub it into their gums. Others dissolve it in water and inject it or inject a combination of cocaine and heroin, called a speedball. Another popular method of use is to smoke crack cocaine [23].
Cocaine increases levels of dopamine in brain circuits controlling pleasure and movement. Short-term effects include vasoconstriction, hypertension, tachycardia, nausea and abdominal pain, extreme happiness, increased energy and body temperature, irritability, and paranoia. Long-term effects include epistaxis, higher risk of contracting HIV, hepatitis C, other blood-borne diseases, malnourishment, restlessness, and severe paranoia with auditory hallucinations. Withdrawal symptoms include depression, tiredness, increased appetite, insomnia, vivid unpleasant dreams, slowed thinking and movement, and restlessness.
While no government-approved medicines are currently available to treat cocaine addiction, researchers are testing some treatments, including disulfiram, modafinil, and lorcaserin (used to treat obesity). Furthermore, cognitive behavioral therapy (CBT), community reinforcement approach, contingency management, or motivational incentives, the matrix model and step facilitation therapy are psychological approaches also used.
21.2.4 Heroin and Prescription Opioids
In the decades before 1990, physicians were criticized for undertreating pain. In the late 1990s, however, there was a paradigm shift. Pain came to be referred to as the “fifth vital sign,” and physicians were encouraged to address and aggressively treat pain [24]. Since that time, opioid prescribing, along with opioid sales, and associated complications have increased worldwide. The current state of opioid therapy and abuse continues to increase the tension between the twin challenges of opioid therapy for chronic pain and its abuse leading to dependency, addiction, hyperalgesia, and death among other various complications [25]. Therefore, opioid abuse has become a significant public health problem globally, specifically in the USA and Canada. Estimates suggest that more than 10% of chronic pain patients misuse opioid analgesics. Furthermore, in the USA from 1999 to 2011, consumption of hydrocodone more than doubled and consumption of oxycodone increased by nearly 500% [26]. During the same time frame, the opioid pain reliever-related overdose death rate nearly quadrupled. According to the US Centers for Disease Control and Prevention (CDC), the unprecedented increase in consumption has led to the “worst drug overdose epidemic in US history” [26]. Given the magnitude of the problem, in 2014 the CDC added opioid overdose prevention to its list of top five public health challenges.
Possible reasons for this “opioid epidemic” have been proposed (Table 21.2) [27].
Physician related | Inadequate and inaccurate training on opioid pharmacology and risks |
Lack of access to multidisciplinary chronic pain care | |
Ease of prescribing opioids compared to other chronic pain therapies | |
Patient related | Strong appeal of immediate pain relief provided by opioids |
Focus on pain rather than psychological distress as a treatment target | |
More value placed on pain relief that functional improvement | |
Society and health system related | Acceptance of right to pain treatment and interpretation of this right in terms of access to opioid therapy |
Better insurance coverage for medication than for other chronic pain therapies | |
Aggressive marketing of sustained-release opioids by pharmaceutical companies |
Furthermore, the problem of co-occurring chronic pain and opioid addiction involves a cycle of behavioral escalation in which nociception triggers pain hypervigilance and catastrophizing, amplifying pain with emotional anguish (Fig. 21.3) [28]. Recurrent self-administration of opioids in response to pain and negative emotions results in associative learning processes that bias attention toward opioid-related cues, strengthening the habit of opioid use despite tolerance to opioid analgesia. This downward spiral may ultimately result in mindless, uncontrolled opioid use and addiction.
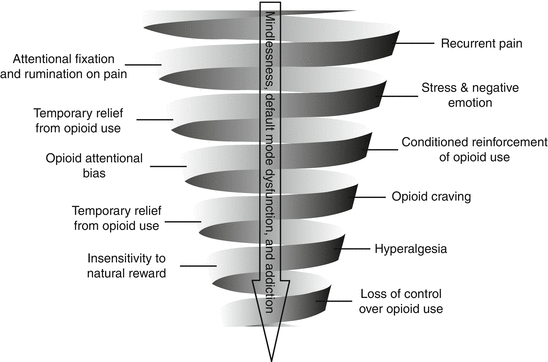

Fig. 21.3
The downward spiral of chronic pain and opioid addiction. From Garland et al. [28] with permission
Opioids are a class of drugs that include the illegal drug heroin as well as powerful analgesics available legally by prescription, such as morphine, codeine, hydrocodone, hydromorphone, oxycodone, buprenorphine, fentanyl, or methadone. Tramadol is also used, although it is not only an opioid agonist but also an adrenaline and serotonin reuptake inhibitor at the spinal level. These drugs are chemically related and interact with opioid receptors (mu, kappa, and delta) on nociceptors at peripheral, spinal, and brain levels. The mu receptor is primarily responsible for the analgesic and euphoric properties of opioids, and many of the opioid analgesics in use today are full mu agonists (morphine, hydromorphone, oxycodone). Buprenorphine is a partial mu agonist that was approved as an office-based treatment for opioid dependence. Because it is a partial agonist, there is less risk of overdose, and long-term use is associated with less severe withdrawal symptoms [24].
Opioid analgesics are generally safe when taken for a short time and as prescribed by a doctor, but they are frequently misused (taken in a different way or in a greater quantity than prescribed or taken without a doctor’s prescription) because they produce euphoria in addition to pain relief [29].
Opioids are available in a wide range of formulations: short-acting orally administered opioids (e.g., immediate-release morphine, hydromorphone, codeine, fentanyl, hydrocodone, and oxycodone) with a rapid onset of action (10–60 min) and a relatively short duration of action (2–4 h), used for acute or breakthrough pain, and extended-release or long-acting opioids with a slower onset of action (30–90 min) and a longer duration of action (4–72 h), typically used for chronic pain conditions [24]. In an attempt to reduce the risk of opioid abuse, PO formulations containing both a strong opioid and an opioid antagonist have been developed in the USA [30]:
Suboxone® (buprenorphine-naloxone) given SL for opioid dependency.
Embeda® (morphine-naltrexone).
Targinact® (oxycodone-naloxone).
OxyNal® (oxycodone-naltrexone).
Specific recommendations to enhance efforts to prevent opioid abuse include [25] (1) prescriber and patient education, (2) careful initiation of opioid therapy in acute pain with limited duration therapy, (3) appropriate initiation and maintenance of chronic opioid therapy, (4) prescription drug monitoring programs, (5) opioid overdose prevention strategies, and (6) expansion of access to medications for addiction treatment and use of abuse-deterrent technology. In addition, best-practice recommendations from a variety of professional societies recognize the need to balance the benefits of opioids in managing pain with the potential risks conferred, particularly by chronic use. Recommendations support the universal application of risk mitigation strategies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






