Fig. 28.1
Case of a 68-year-old diabetic gentleman with severe tissue loss and Duplex ultrasound evidence of a popliteal artery occlusion at the below-the-knee segment. Successful revascularization was performed, with restoration of palpable pedal pulses and excellent angiographic results, fully percutaneous and in an office-based laboratory in Tucson, Arizona. Such highly selected cases can be safely performed in the outpatient setting, with comparable morbidity as in the hospital venue, and significantly less financial charges to the health care system
This change was not exempt from controversy. Critics cite that, since the actual practitioners who staff them own most of these OBLs (and among them, practitioners belong to multiple subspecialties, i.e., interventional radiology, interventional cardiology, vascular surgeons), the financial incentives inherent to owning an OBL have been alleged to affect practitioner behavior. In our personal opinion, we strongly believe that a key requisite to embark into such a financial and professional endeavor, the high moral and ethical behavior that the practitioners abided while they use to perform procedures in the hospital setting need to remain absolutely unchanged when changing operating room scenarios, in line with the Hippocratic Oath. As important of a requisite, is a full knowledge of the natural history of PAD, so that the indication to intervene is based on solid scientific foundations, which could never be questioned. This requires appropriate education of vascular interventionists, regardless of the subspecialty that these practitioners are certified on. Lastly, external peer-review systems and internal measurements of safety and compliance need to be in effect to guarantee adhesion to standards of care.
As strong as our beliefs above, we feel that a vascular surgery practice can strongly benefit from office-based procedures. Ideal Duplex scanning performed by properly trained technicians along with technical advances in endovascular tools allow vascular interventionists to safely and effectively perform arterial and venous peripheral interventions in the ambulatory setting [11]. They allow providers to better manage their schedules, thus permitting scheduling more cases per day while improving the quality of life of the practitioners; as well as significant savings to the health care system, since fees are paid directly to the provider, bypassing overhead costs charged for the exact same procedure in a hospital setting. Several publications and the authors’ own experience have attested that carefully selected peripheral vascular interventions can be performed in the outpatient setting with low complication rates [11].
Advances in Aortic Diseases
Aneurysmal Disease
Aneurysms are arterial dilations to more than 50 % of the diameter of adjacent non-diseased proximal or distal artery. Although aneurysms are found throughout the arterial vasculature, infrarenal AAAs are the most common. They represent the 13th leading cause of mortality in the USA in men older than 65 years [12]. Their etiology is an area of ongoing research. It is thought that there is an initial vessel wall insult (possibly atherosclerosis or smoking-related damage) followed by a pathophysiologic response that results in vessel wall weakening and dilation [13]. Matrix metalloproteinases (MMPs), which are enzymes that degrade arterial wall matrix molecules responsible for arterial wall integrity, are also thought to play a key role in AAA formation and progression [14]. More rarely, AAAs are secondary to autoimmune and collagen-vascular diseases.
Because of the high mortality associated with aneurysm rupture, newly developed guidelines recommend a one-time screening for AAA in males who ever smoked between the ages of 65–75 years old [15]. Current Society for Vascular Surgery (SVS) guidelines suggest surgical repair (open or endovascular) for abdominal aneurysms with a diameter of 5.5 cm and thoracic aneurysms with a diameter 6.5 cm or if the aneurysm has a rapid growth of >1.0 cm/year [16].
Repair of AAAs include both endovascular and open surgical options. However, since Parodi and colleagues placed the first endograft for repair of an AAA in 1991 [17], adoption of this technique has been rapid—over 80 % of AAAs are now repaired endovascularly in the USA.
Whereas no major technological advances have been reported during the last century in the open aneurysm repair technique, major improvements in the endovascular arena have been reported. Successful placement of an aortic endograft depends on: having adequate arterial access; having adequate proximal and distal landing zones for the graft to interface with arterial wall and; and having ideal aneurysm characteristics, specifically the angle of the neck and the amount of wall calcium and thrombus. Improvements in these key factors that govern successful aneurysm repair are listed and explained herewith:
Access Vessels: An exclusion device must fit through an access vessel (primarily the common femoral and iliac arteries), which limits treatment because of access vessel diameter, tortuosity and calcification. The average external iliac artery of men is 9-mm and women is 7-mm. To accommodate, device manufacturers have evolved towards increasingly streamlined products with a smaller profile (smaller diameter). For instance, in November 2011, The US Food and Drug Administration (FDA) approved a stent graft system that provides patients with small arteries the option of endovascular AAA repair. The Ovation Abdominal Stent Graft System (20 millimeter diameter), manufactured by TriVascular Inc. (Santa Rosa, CA) (Fig. 28.2), uses a narrow delivery system, of about 4.7 mm in diameter. This new device differs from other stent grafts in that a portion of the metal stent is replaced with ring-shaped channels, which are injected with a polymer after the device is in place in the aorta, expanding the endograft against the aorta to create a seal.
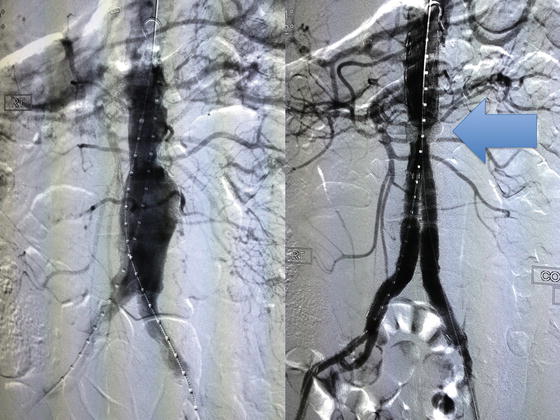
Fig. 28.2
This is a 78-year-old male patient who had a history of a 5.8-cm infrarenal abdominal aortic aneurysm, who had successful placement of an endovascular percutaneous placement of an Ovation Abdominal Stent Graft System (TriVascular Inc., Santa Rosa, CA) by the authors. The ring-shaped channel can be seen inflated with polymer (blue arrow), providing adequate seal for the endograft
This is a very useful addition to the already existing techniques to deal with poor access vessels, including conduits sutured into the common iliac artery or endoconduits lining the native vessel. In the former technique, extra-anatomic prosthetic conduits can be anastomosed to more proximal vessels, such as the common iliac artery, to provide for device delivery. The distal end of the conduit is then often anastomosed to the common femoral artery, which adds the benefit of bypassing often diseased or occluded iliac vessels. With the latter technique, “endoconduits” provide an entirely endovascular option when covered stent grafts with a sufficient diameter are deployed in the common or external iliac arteries to provide conduit for graft access. This technique developed by the vascular group in Malmo, Sweden, is referred to as “crack and pave” because vessels are lined with “endoconduit” and balloon angioplasty then “cracks” the native vessel to allow sufficient diameter for device passage [18].
Proximal Landing Zones: Certain proximal landing zones are compromised by acute neck angulation or neck lengths that are short but not so short as to require fenestration or snorkel, as we will describe later. In such instances, some endorse fixation of the endovascular exclusion device via tacking to the arterial wall. Endofixation using endoanchors is thought to increase the force of fixation and decrease stent graft shifting. The Heli-FX™ EndoAnchor System (Aptus Endosystems, Sunnyvale, CA) is thought to improve fixation and endograft sealing within the infrarenal aortic neck, mimicking a surgical anastomosis. As a results, the force required to displace an endograft is higher, potentially: (1) diminishing the risk of proximal aortic neck complications with difficult aortic necks; (2) treating endoleaks and migration when these complications occurred with a previously placed endograft; (3) increasing eligibility for EVAR to those with more complex aortic neck anatomy, and; (4) improving the durability of endovascular repair through a more robust attachment between the endograft and the aorta. In a recent trial that implanted EndoAnchors in 319 patients with type IA endoleaks, 91 % of endoleaks resolved and at the end of published follow-up, 95 % of patients did not require additional intervention [19]. Results of the ANCHOR prospective, multicenter registry of EndoAnchors for type Ia endoleaks and endograft migration in patients with challenging anatomy [19]. Inadequate neck lengths are seen with juxtarenal and pararenal aneurysms. In such cases, sufficient sealing zones could be created with coverage one or two renal arteries or in some cases large accessory renal arteries. To accomplish this and preserve renal blood flow, fenestrated grafts can be deployed, or alternatively the “snorkel” technique is used when appropriate. For this technique, a covered stent graft is deployed into the artery to be preserved and the proximal end is advanced to above where the top of the AAA exclusion graft will need to be deployed. As such, blood flow is maintained to the preserved artery via a “snorkel.” The snorkel technique is a 95 % patency at 2 years in experienced hands. Direct comparison of snorkel vs fenestrated EVAR (fEVAR) has not been made, but preliminary results from both techniques suggest both have adequate short-term outcomes [20, 21].
Neck Configuration: Aneurysm neck angulation can prevent endograft devices from maintaining arterial wall approximation, which leads to endoleaks and aneurysm sac pressurization. In addition, as aneurysms undergo natural remodeling after EVAR; neck angulation can increase and lengthen which often results in endoleak creation. Required neck angulation has typically been considered <60°, but, increasingly, devices are able to conform to more acute neck angles. Last February 2013, the FDA completed its review of the Aorfix™ AAA Flexible Stent Graft System (Lombard Medical, Didcot, UK). This new device is a highly conformable modular nitinol and polyester stent which combines circular and helical structures that provide the flexibility needed to treat tortuous aortic anatomies. It is indicated for the treatment of patients with abdominal aortic and aorto-iliac aneurysms having vascular morphology suitable for endovascular repair, including infrarenal aortic neck angulations including those up to and including 90°.
Distal Landing Zones: During EVAR, the common iliac artery represents the distal site for stent-graft implantation. The benefit of EVAR could be compromised if an adequate distal seal is not obtained. Concomitant common iliac artery aneurysms are present in 15–40 % of patients with AAA [22]. One solution is to deploy stent-graft extensions into the external iliac artery, but this is thought to compromise the long-term patency of the repair [23]. Furthermore, IIA coverage can result in buttock claudication and/or erectile dysfunction. The actual frequency of such complications is not well defined. In younger populations (with fewer collaterals and who have greater oxygen demand because they are more active), these complications can occur in 30 % of single vessel coverage and 35 % if both hypogastric arteries are covered [24]. But the frequency of buttock claudication in the majority of patients is much lower, but still occurs.
The distal seal zone is quite important for successful AAA exclusion [25, 26]. In fact, difficulties with the distal landing zone represent a more important reason for reintervention than those with the proximal zone. Progressive iliac artery aneurysm formation and iliac limb thrombosis are among the most important reasons for reintervention in a series of 832 EVAR patients [25]. Hypogastric arterial preservation can be achieved to good success using branched endografts that provide flow both into the hypogastric and the external iliac artery [27]. Another useful technique is the “bell-bottom,” where the distal graft flares to create wall apposition with an aneurysmal distal common iliac artery [28].
Next, we present a succinct review of more advanced techniques involved in EVAR. AAA anatomy dictates the feasibility and difficulty of their repair, and multiple adjunctive procedures have evolved during the last century to expand the number of aneurysms amenable to EVAR.
Percutaneous EVAR (pEVAR)
Open exposure of femoral vessels has historically been the preferred method to gain arterial access for AAA endograft deployment because percutaneous access resulted in high complications rates including bleeding and iliofemoral dissection compared to open exposure [29]. Open exposure requires approximately 5–8 cm groin incisions (often bilaterally) and is a safe way to access vessels with direct arterial control. However, this technique results in a small (~1–2 %) but present risk of wound infection. Two factors have allowed for increased utilization of pEVAR (Fig. 28.3): exclusion devices with smaller profiles and improved percutaneous arterial closure devices [30]. A recent multicenter RCT determined pEVAR has decreased surgical site infections and no increase in access vessel complications. However, the study excluded “hostile” groins [31]. Femoral artery calcification is a major predictor of pEVAR success because calcifications can prevent closure devices from proper functioning [32]. In summary, pEVAR vs. open arterial exposure is the surgeon’s preference, but preparedness for open cut-down is necessary.
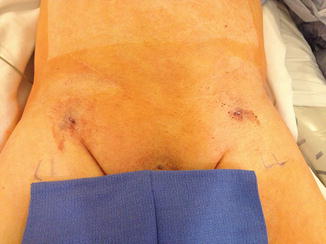

Fig. 28.3
Postoperative photography of a patient after successful percutaneous endovascular aneurysm repair. His body habitus, lack of calcification of femoral vessels, and a small profile endograft made him a perfect candidate for a completely percutaneous approach
Fenestrated EVAR (fEVAR)
The proximal aortic neck remains an important limitation of endovascular treatment of AAAs. Roughly 20 % of aneurysms are not candidates for standard endovascular repair due to poor proximal neck anatomy [33]. As discussed above, appropriate infra-renal landing zones of 10–15 mm is required for proximal graft seal. A recent meta-analysis including 1,559 patients retrospectively reviewed found that patients with hostile AAA neck anatomy had a fourfold increased rate of Type I endoleak and a ninefold increase in 1-year aneurysm-related death [34]. Endografts can be fenestrated for renal, superior mesenteric and celiac artery accommodation. This technique started with graft modification (cauterizing a hole in a prefabricated graft), but pre-fenestrated grafts are now available at specific study centers in the USA. Useful comparison between fEVAR and open repair of juxtarenal and visceral aneurysms are not useful because the patient populations in the two arms are so disparate [35]. Assuming that patients selected for fEVAR have greater comorbidities than those who are candidates for open repair, the results that indicate equivalent 30-day mortality (4.1 %) between the two groups is encouraging [36]. Although data is limited, the technical feasibility of fEVAR has been shown and the method is increasing.
EndoVascular Aneurysm Sealing (EVAS) system
It is thought that up to 40 % of infrarenal AAA are not suitable for EVAR due to hostile proximal neck anatomy (e.g., highly angulated, dilated, short, or encroaching on or involving the renal arteries [12, 37, 38]). In most EVAR studies, the infrarenal non-aneurysmal neck length and angulation to the aneurysm sac requirements are ≥15 mm and ≤60°, respectively; shorter or more angled necks have been reported to increase the risk of migration, endoleaks and associated need for intervention [38, 39]. When selecting the specific stent graft to be used for EVAR, the characteristics of the graft must be considered in light of the patient’s anatomic and physiologic characteristics. Most of the currently available devices seal aneurysm by proximal and distal fixation of the stent graft by either active fixation (anchoring pins) or stent oversizing for increased radial force thereby achieving seal and excluding the aneurysm sac. Greater than 25 % of subjects develop an endoleak (mostly type II, due to back-bleeding of branch arteries within the aneurysm sac) within the first 2 years following EVAR [40].
Endovascular aneurysm sealing (EVAS), the Nellix® system (Endologix, Irvine, CA) was designed to exclude the aneurysm with two ePTFE balloon-expandable covered stents creating pathways to each common iliac artery. In addition, the system deploys an “Endobag” filled with polymer that surround the two stents and fills the aneurysm sac (Fig. 28.4). This is a unique, “out of the box thinking” kind of device. In theory, this Endobag promotes graft stability, prevents aneurysm degeneration and diminishes possibility of all types of endoleaks. A clinical trial has been performed to assess the safety and effectiveness of the first generation Nellix system for EVAR and results serve as the basis for CE Mark approval in October 2012. Initial results showed that the Nellix System is quite versatile in treating a variety of AAA anatomies, including those with common iliac artery involvement bilaterally. An ongoing trial with the Nellix System in a broader group of institutions and physicians was then begun to assess its safety and effectiveness. This is a multicenter, prospective, single arm clinical study. Subjects with infrarenal AAA who are suitable candidates for EVAR were considered for enrollment. Up to 30 sites in the EU, Canada, and the US enrolled patients for endpoint analysis. The enrollment phase has closed and now the trial is in the follow-up stage.
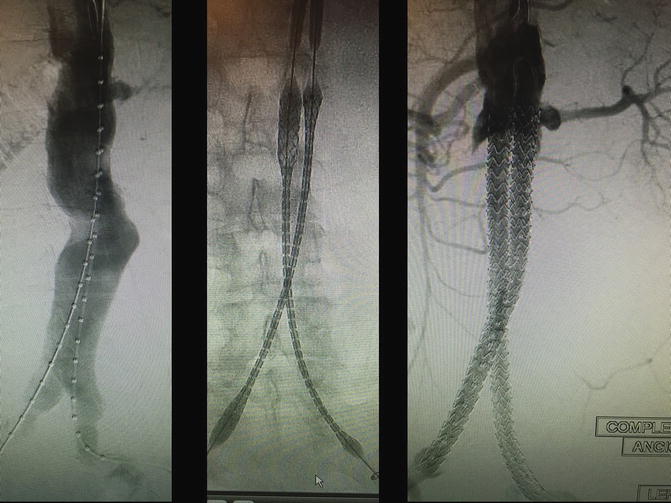

Fig. 28.4
These are intra-operative angiographic images of a patient enrolled in the EVAS Nellix® (Endologix, Irvine, CA) trial by the authors. Patient is an 83-year-old Caucasian male with a 6.8-cm infrarenal abdominal aortic aneurysm, with ideal features for an endovascular repair (left panel). The two e-PTFE balloon-expandable covered stents of the Nellix system are being deployed (middle panel), with the final result showed in the right panel, with successful exclusion of the aneurysm sac
In summary, AAAs are an important disease and a common cause of death in our aging population, especially in those who have ever smoked. Disease treatment is aimed at prevention of AAA rupture, and the techniques to achieve this are ever increasing and complex. The available technology generally outpaces our ability to thoroughly evaluate it and determine their long-term (5–10 year) success. As such, this requires experts who stay abreast of technology and consider such technology with a discerning and critical eye using years of experience with aneurysm anatomy and physiology to make judgments of how best to deal with each patient’s anatomy.
Acute Aortic Syndromes
Endovascular Management of Ruptured AAA
One of the most immediately life-threatening challenges in vascular surgery is repair of a ruptured aneurysm involving the aorta and/or iliac vessels. Classically this has been addressed with an open abdominal approach and a perioperative mortality rate of nearly 50 % [41]. While this has been slowly decreasing at about 3.5 % per decade, an exciting development in the twenty-first century is the increasing body of data on the use of endovascular grafts and devices for not just elective aneurysm repair, but also in cases of rupture.
Multiple centers have demonstrated and published the feasibility of endovascular repair of ruptured aneurysms in the emergent setting with reduced rates of perioperative mortality [42–46]. By analyzing data from the National Surgery Quality Improvement Project (NSQIP) database, a real-world comparison of open repair vs. EVAR could be made [47]. While the 30-day mortality risk was increased with open repair vs. EVAR, this was not statistically significant in this study. However, those treated with open repair had significantly higher rates of blood transfusion, overall morbidity and rates of pulmonary adverse events.
Utilizing femoral access for endovascular techniques as opposed to open laparotomy results in smaller incisions and lower rates of morbidity, but one of the limitations of endovascular repair instead of open repair are endoleaks that result in continued pressurization of the aneurysm sac. This may be particularly challenging in the emergent setting as device options may be more limited and the usual planning is more rushed. Along with a good stock of endografts for treating ruptures, the development of techniques to deal with endoleaks is helpful an assuring depressurization of the aneurysm. If needed for seal, embolization of the internal iliac arteries is more easily achieved before deploying a device across them as is sometimes performed in elective repairs, and type II endoleaks are not always easy to cannulate and occlude. However, published techniques such as direct sac puncture via translumbar approach appear to provide viable solutions to these problems [48, 49] Still, quick planning and an armamentarium of wires, catheters, embolization coils and vascular plugs can make control of these branch vessels feasible even in the setting of rupture.
Type B Aortic Dissections
The gold standard in the therapy of acute type B Aortic dissections (AAD) remains medical therapy. Despite progress in surgical techniques for open repair, the morbidity and mortality associated to this procedure remains high. Thoracic endovascular dissection repair (TEDR) has emerged as a less morbid procedure with potential clinical benefit [50].
Recently the task force for the diagnosis and treatment of Aortic disease of the European Society of Cardiology agreed on TEDR as the treatment of choice for complicated ADD. The term complicated refers to persistent or recurrent pain, uncontrolled hypertension despite full medication, early aortic expansion, malperfusion and signs of rupture (hemothorax, increasing periaortic and mediastinal hematoma) [51].
In uncomplicated patients, there seems to be a trend toward more aggressive TEDR. The intention of expanding interventional therapy in this subgroup is to stabilize the dissected aorta and prevent late complications by inducing aortic remodeling. From the technical standpoint, the operator should attempt to close the proximal intimal tear by implanting a stent-graft and redirecting blood flow from the false lumen to the true lumen [52].
The INSTEAD trial randomized a total of 140 patients with subacute (>14 days) uncomplicated ADD to receive either best medical management or endografting. Two-year follow-up results indicated that TEDR was an effective tool regarding successful Aortic remodeling. The TEDR group had 91.3 % vs. 19.4 % remodeling in the medical cohort. That said, TEDR demonstrated no clinical benefit over medical therapy from the survival standpoint (88.9 ± 3.7 % with TEDR vs. 95.6 ± 2.5 % with optimal medical therapy; p = 0.15) [53].
Upon extended follow-up and statistical analysis at 5-years (INSTEAD-XL trial) the aorta-related mortality (6.9 % vs. 19.3 %; p = 0.04) and disease progression (defined as aneurysmal dilation or further dissection) were significantly lower on the TEDR subgroup. There was no difference in overall mortality [54]. The IRAD registry, a multicenter international ADD registry, has also published similar results and has further identified factors that may help risk-stratify these patients and tailor the aggressiveness of the therapy [55, 56].
The role of preventive TEDR in uncomplicated but high risk ADD is yet to be definitively proven, but the following are clinical scenarios that selected authors advocate merit a more aggressive primary-TEDR approach: primary entry tear with a diameter greater than 10 mm; primary entry location at the lesser curvature of the arch (“concave” side); total Aortic diameter greater or equal to 4 cm; false lumen diameter greater or equal to 22 mm; partial false lumen thrombosis and Marui Fusiform Index greater than 0.64; Marui et al. developed this indicator: Maximum total diameter (including the false lumen) of the descending Aorta divided by the sum of the diameters of the Aortic arch and descending Aorta at the level of the pulmonary artery [57, 58].
Advances in Peripheral Arterial Disease
An estimated 12–20 million people in the USA alone have PAD [59]. PAD symptoms and signs include walking impairment (fatigue, aching, numbness, and pain), ischemic pain at rest, and chronic non-healing wound. The most common presenting symptom of lower extremity PAD is ambulation-induced muscle pain, or claudication. PAD is characterized by occlusive lesions in the lower extremity and portends an increased cardiovascular risk. [60]. Atherosclerotic lesions occur throughout the arterial tree in vessels from the aorta to pedal arteries. The location of arterial disease has important clinical implications for both prognosis and management [61].
Patients with critical limb ischemia (CLI) typically have multilevel arterial occlusive disease and significant comorbidities that increase their perioperative risk. Successful revascularization and limb salvage traditionally required lengthy, often staged interventions with significant associated morbidity and mortality. Endovascular techniques present an attractive alternative to open interventions especially in high-risk patients. Balloon angioplasty and/or stenting for TASC A and B (and perhaps for TASC C) iliac lesions have proven very good patency rates and have become the preferred initial treatment [61]. CLI, however, has long been considered an indication for bypass surgery [62]. The growing experience with endovascular techniques has helped to better identify patients and lesions that are best-treated by percutaneous interventions. Kudo et al. [63] described this change in practice over a 12-year period showing that balloon angioplasty could be the procedure of choice for CLI therapy.
Advances in the Diagnosis and Assessment of Peripheral Arterial Disease
The etiology of PAD in the vascular practice appears to be undergoing a change along with the changing population of the USA. The usual vascular risk factors are still present (i.e., age, smoking, hypertension, hyperlipidemia); however, the prevalence of diabetes in the USA and around the world has been increasing in the twenty-first century. The sensory and autonomic neuropathy, increased rates of chronic kidney disease, immunosuppressive and wound-healing inhibitory effects of diabetes make it much more challenging than the pure-ischemia model described in the term critical limb ischemia originally defined in 1982 [64]. Recognizing these complexities, the authors of that paper explicitly excluded diabetics from assessment by this method. Diabetes also appears to change the pattern of disease from the classic proximal distribution (aortoiliac and femoropopliteal) to a more distal distribution of tibial vessels and intrinsic foot vessels. This often results in medial calcinosis of these vessels making it difficult to assess severity of disease and response to treatment with conventional methods of ankle-brachial index and toe pressure as medial calcinosis causes false elevation of these pressures and intrinsic foot disease combined with tibial occlusions may cause regionalized ischemia of the foot even with normal pressure in one of the other tibial vessels. For this reason, alternative methods of assessing tissue perfusion are being investigated. One of these methods is using Indocyanine Green (ICG) dye injected intravascularly and visualizing the fluorescence using a charge-coupled device camera. The dye stays intravascularly and the intensity of the fluorescence in the tissue corresponds to the degree of perfusion of the tissue. It therefore does not assess blood flow based pressure but is an assessment of end-perfusion. This technology has been used for decades in ophthalmology, cardiothoracic surgery, plastic surgery, liver transplantation, bowel resections and surgical oncology. Its use in assessing foot perfusion in patients with PAD and foot wounds is still in the early stages but multiple series have been published in the USA and around the world on this topic. It requires an IV to test, which is more invasive than traditional noninvasive tests of ABI/Toe pressure and duplex ultrasound; however, it is available for use even when traditional noninvasive tests are unreliable or unobtainable due to medial calcinosis, active wounds, prior amputations or other conditions. Multiple criteria have been proposed as surrogates for adequate perfusion for healing [65–67] but as of now, no accepted standard has been adopted.
Another technology for monitoring tissue perfusion is implantable micro-oxygen sensors. A first-in-man study has been published recently based on technology from PROFUSA, Inc. (South San Francisco, CA) [68]. The eventual hope is that an implantable, biodegradable tissue oxygen sensor could be implanted in the foot & serial monitoring be done either at the physician’s office or via an app on a hand-held device, allowing earlier detections of change that may signal worsening arterial stenosis or impending occlusion at a time when it is easier to repair than when the vessels have occluded. While this technology is still well away from mass use, it may fill a gap left by current vascular assessments.
Advances in the Therapy of Peripheral Arterial Disease
Aortoiliac Arterial Disease
Endovascular therapy of the aorto-iliac system has far developed since the initial results by Kichikawa et al. in 1990 [69]. The Dutch trial further analyzed the impact of metal scaffolding, concluding that simple (TASC A/B) lesions respond well to plain old balloon angioplasty reserving stenting as a bail-out plan [70].
The widespread use of endovascular therapies has lead operators to tackle more complex lesions (TASC C/D). Within this specific complexity, some of the preliminary data suggests an advantage of PTFE-covered stents over BM-stents [71]. Sabri et al. concluded that the use of covered balloon-expandable kissing stents for atherosclerotic aortic bifurcation occlusive disease provides superior patency at 2 years when compared with bare meal balloon-expandable stent in a small number of patients [72].
In 2011, the COBEST (Covered versus balloon expandable stent trial) trial revealed its early data. Aortoiliac lesions treated with a PTFE-covered stent were significantly more likely to remain free from binary restenosis than those that were treated with a bare-metal stent (hazard ratio [HR], 0.35; 95 % confidence interval (CI), 0.15–0.82; p = 0.02). Freedom from occlusion was also higher in lesions treated with covered stents than in those treated with a bare-metal stent (HR, 0.28; 95 % CI, 0.07–1.09); however, this did not reach statistical significance (p = 0.07) [73].
Femoropopliteal Arterial Disease
Contemporary treatment of femoropopliteal occlusive arterial disease is most frequently via an endovascular approach. When comparing inpatient vascular procedures in the USA from 1997 to 2008, open peripheral reconstruction declined by 24.5 %, whereas percutaneous interventions dramatically increased by 296.8 % [74]. Unfortunately, the combination of a wide array of available endovascular tools, inconsistent outcomes data, and differing training paradigms, have produced uninformed and highly variable endovascular practice patterns.
The lack of consensus regarding reporting standards leaves the practitioner with inadequate data to formulate evidence-based clinical decisions. Some authors have attempted to create uniformity [75, 76] but there are as yet no broadly accepted and applied practice standards. Moreover, most of the randomized controlled data available to date are industry driven.
Restenosis of femoropopliteal therapy has been certainly the main concern during the past 10 years of practice. There is not one device that has proven to be far superior to others, yet there are evolving technologies that have added value and a potential role in this segment. Amongst the most notorious advances in the field are: new fracture-free, vasculo-mimetic stents; drug-coated stents; drug-coated balloons; advanced drug-delivery systems; and newer generation atherectomy (plaque debulking) devices.
Fracture-Free, Vasculo-mimetic Stents
The Supera® (Abbott Vascular, Santa Clara, CA) self-expanding, interwoven, nitinol-stent received FDA approval for use in the femoropopliteal early in year 2014. The study that led to the FDA approval is known as the SUPERB registry. The primary patency rate achieved in this pivotal trial was 86.3 % (Kaplan–Meier) with 1-year freedom from reintervention at 89 % and 3-year freedom from reintervention (TLR) at 83 %, despite having 45 % of patients with severe calcium and 25 % with total occlusions.
A subset analysis of the SUPERB trial was conducted to determine the impact of lesion length on restenosis. In both short lesions (3.5 cm) and long lesions (12 cm), the percentage of patients without restenosis was the same at 88 %, which was dramatically different than other investigational device exemption trials such as STROLL (S.M.A.R.T.® Control®, Cordis Corporation, East Bridgewater, NJ) or DURABILITY II (EverFlex™, Covidien, Minneapolis, MN), which showed a decline in patency as lesion lengths increased.
The main issue with inter-woven technology is the potential to mis-deploy resulting in an elongated or compressed conformation. In proper deployments of Supera® (Abbott, Abbott Park, IL) (nominal length deployments), primary patency was 90.5 % at 12 months. In addition, 97 % and 94 % freedom from reintervention rates were achieved at 1 and 3 years, respectively, in nominal deployments. Lower primary patency and freedom from reintervention rates were associated with severely elongated deployments.
Drug-Coated Stents
One of the major factors causing recurrent stenosis in peripheral artery interventions is the development of neo-intimal hyperplasia. This is an overgrowth as a result of the vessel’s response to injury. Original investigations into this problem were conducted in the coronaries, and the first drug-eluting stent (DES) was approved in Europe in 2002 with FDA approval in 2003 initially for treatment of in-stent restenosis [77]. Drug-eluting stents cause a time-released local application of a drug (generally a chemotherapeutic drug such as sirolimus, paclitaxel or everolimus) to the tissue abutting the stent in order to inhibit the inflammatory response and neointimal hyperplasia, thereby reducing rates of restenosis and thrombosis. Since initial approval, the use of drug-eluting stents has expanded profoundly in coronary artery disease.
Given that the same neointimal hyperplasia process occurs in the periphery as well as in the coronary bed, eventual research looked into using drug-eluting stents for peripheral artery disease. The first DES approved for use in the superficial femoral and popliteal arteries was the Zilver® PTX stent by Cook (Cook Inc, Bloomington, IL) [78, 79], which received FDA approval in 2012. The Zilver PTX stent elutes paclitaxel from a polymer-free laser-cut nitinol stent. Dake et al. led the Zilver PTX stent trial which initial results were published in 2011 [78]. This was a prospective, multinational randomized controlled trial and a complementary single-arm study evaluated the 2-year safety and effectiveness of the implant in patients with superficial femoral artery lesions. The trial compared the DES with percutaneous transluminal angioplasty and provisional bare-metal stent placement.
The trial showed an unsurprising superiority of drug-eluting stent over balloon angioplasty in terms of primary patency (74.8 % vs. 26.5 %, p < 0.01). However, it also showed superiority in the use of drug-eluting stent over bare-metal stent in patients who had initial failure of primary angioplasty (83.4 % vs. 64.1 %, p < 0.01). This corresponds to a number needed to treat (NNT) of 5 to prevent 1 re-thrombosis at 2 years. There is an up-front higher cost to the drug-eluting stents, which is one of the reasons why it has not replaced bare-metal or covered-stent technology in the primary treatment of PAD. In addition, the provisional DES group achieved higher sustained clinical benefit (83.9 % vs. 68.4 %, p < 0.05). Two-year freedom from target lesion revascularization with primary DES placement was 80.5 % in the single-arm study and 86.6 % in the Randomized Control Trial [79].
Even though these new-generation devices have proven to be superior to older scaffolds, the presence of a non-absorbable implant still perpetuates a constant inflammatory process that may lead to intimal hyperplasia. Tepe et al. attempted to introduce a new paradigm of care with the publication of the THUNDER trial. The preliminary results of these trial concluded that the use of paclitaxel-coated angioplasty balloons during percutaneous treatment of femoropopliteal disease is associated with significant reductions in late lumen loss and target-lesion revascularization without leaving any implant behind [80].
Drug-Coated Balloons
Another area of ongoing interest is the use of drug-coated balloons (DCBs) for treatment of lower extremity arterial stenosis (Fig. 28.5). The rationale is that if a drug-eluting balloon can provide improved patency results without the need for leaving a stent in place, it might help avoid some of the other problems of stents such as fracture or ongoing stress leading to neointimal hyperplasia. Late in 2014, the FDA approved the Lutonix® paclitaxel DCB (Bard PV Inc, Phoenix, AZ) for use in the USA. The primary composite safety endpoint for Lutonix was noninferiority to PTA. For the primary effectiveness endpoint, utilizing duplex scan evaluation with a lax peak-systolic velocity (PSVR) of 2.5, Lutonix demonstrated superior 12-month patency rates to PTA using Kaplan–Meier estimates (73.5 % vs. 56.8 %). However, when using a standard PSVR of 2.0, as used in the SUPERB and ZILVER PTX pivotal trials for primary patency assessment, there was no significant difference between Lutonix and plain old balloon angioplasty (P = 0.13). Moreover, the Lutonix freedom from TLR dataset demonstrated no significant difference with balloon angioplasty alone at 12 months. Despite the lower-than-expected patency rates, Lutonix’s results did show a favorable trend over PTA on several endpoints, suggesting that there is a benefit conferred from the paclitaxel coating [81].
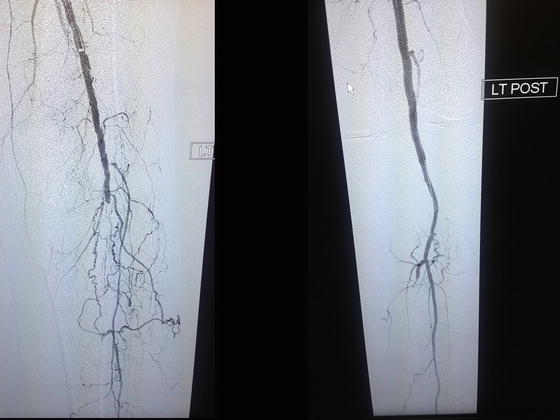

Fig. 28.5
Patient is a 79-year-old diabetic female with advanced gangrene of the foot and severe ischemic cardiomyopathy. Successful endovascular revascularization was performed by using a drug-eluting balloon, with outstanding angiographic results and restoration of palpable pedal pulses
Most recently, in early 2015, the FDA has approved the use of the IN.PACT balloon by Medtronic (Minneapolis, MN), which is a paclitaxel-coated balloon, for the treatment of SFA and popliteal disease [82]. It has been in use in Europe since 2009. The paclitaxel-coated balloon resulted in higher 1 year-patency rate compared to non-coated balloon angioplasty (82.2 % vs. 52.4 %, p < 0.001) and a lower rate of clinically driven target lesion revascularization (2.4 % vs. 20.6 %, p < 0.001). Of note, the patients in this study had predominately claudication as their indication with only 5 % of the DCB arm had rest pain along with 5.4 % of those in the PTA arm along with 0.9 % in the PTA arm with minor tissue loss. Therefore, the lack of amputations eventual amputations in the 1-year follow-up was not surprising. Robust data on other clinical endpoints such as wound-healing and amputation-free survival in patients with tissue loss is lacking for this technology, but further research is ongoing. Combining local pharmacologic effects and biomechanical effects of balloons/stents is on the cutting-edge of PAD treatment.
Advanced Drug-Delivery Systems
Concern has grown as to the potential down-stream loss of cytotoxic drugs in patients with ischemic foot ulcers. Development of novel tools to directly allocate drugs in a safe and efficacious manner is being set in motion. The patented, proprietary and FDA-cleared Bullfrog® Micro-Infusion Device (Mercator MedSystems Inc, San Leandro, CA) delivers drugs directly to the adventitia. Inflation of the balloon safely slides a micro needle through the vessel wall, where agents are delivered and diffuse to bathe the vessel cylindrically (circumferentially, longitudinally and transmurally). Delivering drugs through the blood vessel wall into the perivascular space and adventitia allows for direct, highly controllable and concentrated treatment, minimizing the toxicity of systemic administration, while avoiding the dilution of drug in the bloodstream. The DANCE study—a US-based, multicenter, prospective registry is in the process of recruiting patients to evaluate the use of this device as means to dose steroids to the deep arterial layers.
The TAPAS (“Targeted Adjusted Pharmaceutical Administration System”) catheter (ThermopeutiX® Inc., San Diego, CA) is another newly introduced device, with FDA clearance and CE mark (Fig. 28.6). This device features two occlusion balloons that allow targeted local delivery of any pharmaceutical agent, i.e., chemotherapy drugs, thormbolysis medications, sclerotherapy, anti-inflammatory agents, etc. The distance between the balloons determines the treatment zone, and this can be adjusted from 15 to 300-mm due to its coaxial design. The medication can be aspirated out of the catheter after therapy if desired. Research is planned to prove the safety and efficacy of this technology in the peripheral circulation.
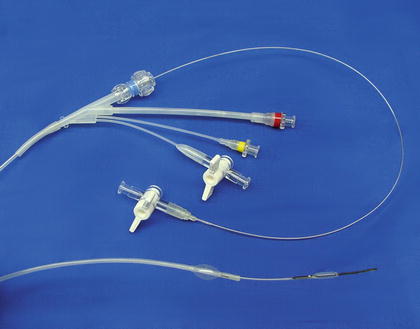

Fig. 28.6
The TAPAS® (“Targeted Adjusted Pharmaceutical Administration System”) catheter. (Courtesy of ThermopeutiX® Inc., San Diego, CA)
New Generation Atherectomy Devices
These have emerged as additional alternatives for plaque debulking. For instance, the Volcano’s Phoenix® Atherectomy System (Menlo Park, CA) is a novel device which has a front-cutting design, with its housing capturing atherectomy debris by a helical blade that cuts inward, mechanism known as “Continuous Mechanical Conveyance.” In this manner, the debulked plaque is removed without vessel suck down or excessive aspiration of blood. It is a single insertion, single-operator device. There is no need to remove catheter to purge the collected material during the procedure. There is no capital equipment component or tableside infusion pump required. The distal cutting element rotates at 12,000 RPM. The cutting element is made of stainless steel alloy with proprietary coating. In theory, the front cutting design eliminates the need to pass the device nosecone through a disease segment prior to treatment. This, along with its mechanism of cutting, capturing and clearing, could potentially reduce the risk of distal embolization. The device has been designed to treat a wide range of disease types, from soft plaques to calcified arteries. In addition, it has the ability to actively deflect its direction, thus being able to cut a lumen larger than the catheter diameter. A small 5 F profile optimizes treatment of disease located below the knee. Lastly, being an over-the-wire device, its pushable design maximizes deliverability. There are several devices, both for femoropopliteal and for tibial applications (5–7 French profile, 1.8–2.4 mm of luminal gain depending on the device used). The femoropopliteal device has a deflecting tip, which allows the interventionist to guide the helical blade as needed. The EASE Study (Endovascular Atherectomy Safety and Effectiveness Study) was designed for the clinical evaluation of the safety and effectiveness of Volcano’s Phoenix® Atherectomy System in atherectomy of the peripheral vasculature in an IDE Study. It was a prospective, single arm, multicenter, FDA-approved IDE study in the USA and Germany. This included lower limb atherectomy with or without adjunctive therapies. This cohort included one-third of patients afflicted with critical limb ischemia and 70 % of the lesions were located at or below the knee level. More than half of these patients were diabetic. With a follow-up at 30 days and 6 months, the Phoenix Atherectomy System proved to be a safe and effective atherectomy solution for peripheral arteries. The study met predefined performance goals in safety and effectiveness; it had a high technical success, (95.1 %) low clinical complications and major adverse events (5.7 % at 30 days). These results were consistent with pivotal trials for predicate devices. FDA clearance was received on January 23, 2014.
Tibial Arterial Disease
The first published successful experience using a retrograde approach to recanalize a tibial vessel was by Iyer et al. in 1990, via cutdown of the posterior tibial artery [83]. The suspected hypothetical benefit of using alternative retrograde wire passage was first well described by Ozawa et al. in 2006 [84]. Although this article exemplifies retrograde chronic total occlusion (rCTO) crossing in the coronary arteries, the fundamental, physiopathologic principles can likely be extrapolated, with some caveats, to tibial-pedal artery procedures. Briefly, the technical hurdle with antegrade guidewire passage is failure to penetrate the proximal cap or failure to accomplish true-lumen distal reentry, primarily due to differential fibrotic cap composition. A complex and hard proximal fibrotic cap will prevent the wire from crossing or quickly divert it into the subadventitial space. In contrast, the distal fibrotic cap is said to be “either very thin or nonexistent” [83]. Moreover, there are potentially invisible residual true lumen channels that taper proximally to their narrowest area, which, as a result, are invisible on angiography. In general terms, the objective of retrograde access is to aid in lesion traversability. Once the lesion is crossed and the wire is secured in the proximal true lumen, most operators recommend recapturing the wire from a proximal, conventional access site and then using it as a rail (a through-and-through configuration that is achieved by establishing the wire from the pedal to the groin access site). Finally, once the lesion is crossed in an antegrade fashion with a support catheter or a balloon catheter, the wire is pulled out of the pedal access site and repositioned in the usual antegrade configuration. Hemostasis of the pedal puncture site is achieved with a combination of simple direct pressure and endohemostasis (inflation at the level of the puncture site of an angioplasty balloon sized one-to-one to the vessel at a low atmospheric pressure for 2–4 min). The development of newer devices with lower profiles has allowed operators to utilize retrograde access not only to cross the lesion, but also to effectively treat it [85].
Retrograde access should be secured close to the area of access artery reconstitution. Long areas of non-diseased vessel will hinder force vectors and minimize wire and balloon pushability. Moreover, vessel spasm is not infrequent, and the interventionalist must always abide by the general principle to minimize unnecessary contact between a healthy vessel and the wire or catheter. With these principles in mind there will be two groups: proximal (or high) and distal (or low pedal) retrograde access.
Initial experience in the 1990s described cutdown as a means of accessing pedal vessels, and although bailout surgical cutdown might be useful on rare occasions, the experienced operator will rarely need to resort to it. More recent published experiences have demonstrated the safety of percutaneous vessel access across a multitude of publications [86]. Recently, an industry-sponsored multicenter registry of nearly 200 patients who underwent rCTO therapy also concluded that the technique was safe and associated with high, initial technical success rates (tibiopedal study sponsored by Cook Medical; unpublished data).
Once the access is secure, there are two basic options: a sheathless or micropedal sheath technique. Historically, complications associated with pedal access were related to large-bore sheaths and these should be avoided [87].
The sheathless technique was used initially and has the theoretical advantage of creating a smaller arteriotomy. Operators secure a long wire in the vessels via the usual Seldinger technique, but then support the wire with either a low-profile balloon or catheter directly through the skin. The drawbacks associated with this are the potential risk of losing access during device exchange and the lack of a port that can be used to administer medications (i.e., antivasospastic drugs) or to inject contrast to obtain angiographic control images.
Current best practice is to secure the access with use of a 2.9-F micropuncture pedal access set. This approach secures low-profile access during the entire procedure. Operators can freely change devices and wires without compromising access, and there is a port to inject medications or contrast. Although most balloons on the market are not compatible with an extremely small inner lumen diameter, the Micro™ 14 (Cook Inc, Bloomington, IL) is a newly approved device that overcomes this hurdle.
Devices for Nonsurgical Management of Peripheral Arterial Disease
Claudication is normally a nonsurgical, medically treated problem. PAD can be improved in many patients with medical treatments [88]. In the absence of heart failure, cilostazol should be considered in patients with claudication pain [88]. Ischemic ulcers are among the most difficult to treat successfully and almost as a rule they typically require surgical interventions for their therapy.
High-pressure, rapid sequence, intermittent pneumatic calf and foot compression (HPIPC) devices apply compression to the foot, ankle and calf using cuffs attached to the leg. This compression regimen simulates the beneficial effects of brisk walking, without pain or tissue trauma. In 1917 Sinkowich and Gottlieb [89] were the first to report the benefits of intermittent compression for the treatment of thromboangiitis obliterans (Burger’s Disease). In 1934, Herman and Reid [90] demonstrated that pneumatic compression improved tissue perfusion in patients with PAOD. Since 1993, several trials on the effects of HPIPC for the treatment of arterial claudication and CLI have been reported [91–94]. In all of these studies the investigators reported significant beneficial effects on walking distance, symptoms and systolic blood pressure measurements on both upper and lower limbs with therapy.
The foot, ankle and calf veins are almost completely emptied in the sitting position by using pressures that are much higher than those typically used in intermittent pneumatic compression (IPC) devices designed for instance, for deep venous thrombus (DVT) prevention. By compressing tissues below the knee, venous blood is almost completely emptied with venous pressures reaching almost zero. The increased arterial–venous pressure gradient results in greater arterial flow. Greater arterial flow alters the shear rate and may stimulate endothelial cell function causing the release of nitric oxide along with tissue factor pathway inhibitors that cause dilation and anticoagulation [95]. Several studies using HPIPC have shown improvement in perfusion, claudication (resting) pain, and wound healing in patients with PAOD and CLI [96–98]. HPIPC therapy appears to significantly improved peak walking time, resting pain, healing rates and physical function in most studies, along with a low device-related complication rate. HPIPC appears to be safe and effective and is an important adjunct to the medical treatment of PAD and CLI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








