(1)
Chennai Breast Centre, Chennai, India
The most important advance in targeted therapy for breast cancer is the ability to target the estrogen receptor(ER) and human epidermal growth factor tyrosine kinase receptor 2 (HER-2) changing the outcome of disease drastically. There is a plethora of the targeted agents in breast cancer that have come up one after another in the recent times. It remains critical to choose a targeted agent keeping in mind its efficacy, biology of the disease, side effects, ideal way of using with or without chemotherapy, and guiding treatment decisions with the help of predictive biomarkers.
There is an upcoming role of dual HER-2 blockage in order to overcome resistance and thus improving the clinical outcomes combining either two drugs of trastuzumab, lapatinib, and pertuzumab. Novel strategies targeting proliferation signaling (mammalian target of rapamycin) does the same thing in hormone receptor-positive advanced breast cancers. Drugs inhibiting angiogenesis including Bevacizumab and targeting DNA repair including polyadenosine diphosphate-ribose polymerase (PARP) inhibitors are still investigational and their current role in breast cancer still remains to be clarified.
Breast cancer alone is expected to account for 29 % of all new cancers among women and is second most common cause of cancer deaths in women in Western world [1]. Breast cancer survival has significantly increased in last two decades owing to better understanding of disease biology and availability of targeted therapy. Targeted therapy refers to drugs that act on specific molecular targets in cancer cells instead of interfering with all rapidly dividing cells. Breast cancer is one of the success story of targeted therapy which began with tamoxifen and other anti-hormonal agents and giving way to trastuzumab and other anti HER-2 therapy and now more recently Bevacizumab, Everolimus, and Olaparib.
Targeted therapies in breast cancer can be broadly classified into tyrosine kinase inhibitors (TKIs) directed at a number of targets (HER-1, HER-2, HER-3, IGF receptor [IGFR], C-MET), inhibitors of intracellular signaling pathways (PI3K, AKT, mammalian target of rapamycin [mTOR], ERK), angiogenesis inhibitors, and agents that interfere with DNA repair. Whereas conventional cytotoxic anticancer agents have major adverse events like nausea/vomiting or myelosuppression in common, targeted therapies are associated with fewer and less toxic side effects than standard chemotherapy or radiation because they cause little or no collateral damage to normal cells. Side effects caused by targeted therapies are directly related to the specific molecular target in normal tissues inhibited or modulated by the specific drug as exemplified by cutaneous toxicity of lapatinib and cardiotoxicity of trastuzumab.
HER-2 Pathway in Breast Cancer and Mechanism of Action of Drugs
HER-2 is a member of erb B epidermal growth factor receptor tyrosine kinase family. Slamon et al. first reported that the HER-2 receptor was overexpressed in 20–30 % of breast cancers [2]. Majority of cases of HER-2 overexpression are caused by amplification of HER-2 gene [3]. Her-2 has no identified ligand thus its open confirmation allows it to dimerize with HER-1, HER-2, HER-3, and HER-4. Amplification and overexpression of HER-2 gene cause activation of PI3K/Akt and Ras/MAPK signal transduction pathways thereby causing cell growth, survival, and cell differentiation (Fig. 43.1) [4].
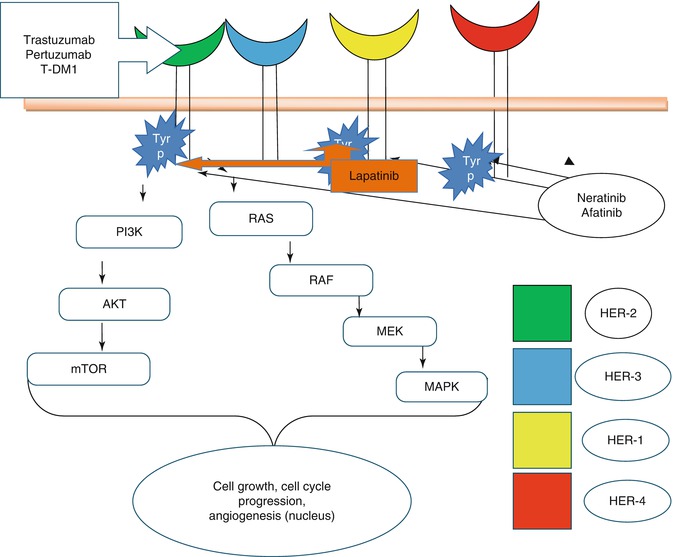

Fig. 43.1
Targeted therapies targeting HER-2
Before the advent of anti HER-2 therapies, HER-2 expression was associated with increased relapse rates and thus increased mortality [2].
Use of HER-2 Targeted Agents in Adjuvant Setting
Trastuzumab in Adjuvant Setting
Trastuzumab is the first HER-2 targeted agent approved for clinical use in breast cancer patients. It is a humanized monoclonal antibody that binds to the extracellular domain of the Her2 receptor. It inhibits signaling by several mechanisms like prevention of HER-2 receptor dimerization, increased endocytic destruction of receptor, inhibition of shedding of extracellular domain, and immune activation [5].
Benefit of Trastuzumab
Slamon et al. conducted a large multinational phase 3 study to compare chemotherapy in combination with trastuzumab to chemotherapy alone in patients with metastatic breast cancer(MBC) in first-line setting in patients with Her2 overexpression. This study showed overall survival (OS) benefit which improved from 20.3 to 25.1 months with addition of trastuzumab [6]. As a result of its success in MBC, six trials were conducted in adjuvant settings. These trials were heterogeneous in terms of patient population, type of adjuvant chemotherapy, timing of adjuvant chemotherapy, schedule of trastuzumab, and duration of trastuzumab. Five out of 6 trials showed risk reduction of recurrence by 40–50 % and risk of death by one-third [7].
Duration of Therapy
Though we have come long way with trastuzumab, still the ideal chemotherapy to be used along with trastuzumab, sequence of giving trastuzumab (sequential or concurrent) with chemotherapy, and optimal duration of trastuzumab is still not known. With the evidence available, optimal duration of trastuzumab seems to be 1 year as recent update of Hera trial suggests that 2 year of trastuzumab was equally efficacious yet more toxic than 1 year of trastuzumab [8]. Another large phase 3 trial designed to evaluate shorter duration of adjuvant trastuzumab failed to show noninferiority of 6 months of therapy as compared to 12 months [9].
Intravenous Versus Subcutaneous Route
The current standard of care for patients with HER-2 positive breast cancer includes intravenous (iv) therapy with trastuzumab over a 30–90 min infusion period. A subcutaneous (sc) formulation of trastuzumab with efficacy noninferior to standard intravenous administration along with a similar safety profile was recently developed that can be administered to patients in approximately five minutes, which would considerably improve the cost-effectiveness and convenience of this treatment [10].
In a recent randomized trial, it was shown that sc trastuzumab was preferred by 92 % patients as compared to only 7 % preferring iv trastuzumab [11].
Toxicities of Trastuzumab
Trastuzumab causes type II cardiac dysfunction usually associated with loss of cardiac contractility and not with myocyte death [12].
Most of the adjuvant trials used stringent inclusion criteria and close cardiac monitoring and thus had lower incidence of symptomatic cardiotoxicity. Overall, the incidence of severe heart failure (New York Heart Association class III or IV) was 0–3.9 % among trastuzumab-treated patients versus 0–1.3 % in patients not receiving trastuzumab.
Lapatinib in Adjuvant Setting
Lapatinib is a reversible inhibitor of epidermal growth factor receptor and HER-2, is approved for use with capecitabine for treating Her2-positive metastatic breast cancer after chemotherapy and trastuzumab failure [13]. TEACH trial randomized HER-2 positive early-breast cancer who had previously received adjuvant chemotherapy but not trastuzumab to receive daily lapatinib (1,500 mg) or daily placebo for 12 months showed that there was no significant difference in DFS between the groups when analyzed in the intention-to-treat population though exploratory analyses restricted to patients who had HER-2 positive disease confirmed by central fluorescence in situ hybridization review suggested marginal benefit with lapatinib in terms of DFS [14]. The Adjuvant lapatinib and/or trastuzumab treatment optimization (ALTTO) study, which is evaluating various anti-HER-2 therapy approaches in breast cancer, has discontinued the single-agent lapatinib arm following a preplanned interim analysis based on 256 event when the study’s data safety and monitoring committee (DSMB) indicated that the lapatinib-alone arm is unlikely to meet the prespecified criteria to demonstrate noninferiority to trastuzumabalone with respect to DFS [15]. As of now single agent lapatinib in adjuvant setting is not recommended while outcome of combination therapy with trastuzumab will be answered by the results of ALTTO trial.
Toxicities of Lapatinib
Lapatinib causes diarrhea, rash, hepatic dysfunction but cardiac dysfunction is very rare as compared to trastuzumab. Among grade 3 and 4 toxicities in TEACH trial lapatinib arm as compared to placebo had higher incidences of grade 3–4 diarrhea (97 [6 %] vs. nine [<1 %]), rash (72 [5 %] vs. three [<1 %]), and hepatobiliary disorders (36 [2 %] vs. 1 [<1 %]) [14]. The drug-associated rash is characterized by inflammatory papules and pustules most often seen on the face, chest, and back and may resemble folliculitis or an acneiform drug eruption and is typically a class effect of drugs that target ErbB-1 receptor.
Neoadjuvant HER-2 Directed Targeted Therapies
As compared to adjuvant chemotherapy, patients enrolled in neoadjuvant chemotherapy trials with trastuzumab are far smaller and most of them have primary end point either pathological response or disease-free survival (DFS) but rarely overall survival (OS). Trastuzumab in combination with chemotherapy has shown increased pathologic response and disease-free survival in both operable and locally advanced breast cancer [16, 17]. In Neoadjuvant Herceptin (NOAH) trial, 235 women with HER-2 positive locally advanced or inflammatory breast cancer were treated with neoadjuvant chemotherapy with or without trastuzumab. Pathological CR (pCR) rate was 38 % in trastuzumab group as compared to only chemotherapy group [17].
Multiple trials have explored the role of lapatinib as neoadjuvant therapy in early-stage HER2-positive breast cancer and concluded that its effectiveness is statistically no better than standard trastuzumab (Table 43.1). In the GeparQuinto study, lapatinib combined with epirubicin and cyclophosphamide proved less effective at producing a pCR prior to surgery than trastuzumab administered with the same chemotherapy regimen [23]. So single agent lapatinib is not recommended in neoadjuvant setting in combination with chemotherapy.
Table 43.1
Neoadjuvant trials of trastuzumab and/or lapatinib with chemotherapy in HER-2 positive breast cancer
Study and regimen | pCR in T (%) | pCR L (%) | pCR H + L (%) | T + L vs. T |
|---|---|---|---|---|
NeoALTTO (n = 455) [18] 6w H and/or L → WP × 12 plus H and/or L | 27.6 | 20.0 | 46.8 | P = 0.0001 |
NSABP B-41 (n = 519) [19] ACX4 → WP × 12 plus H and/or L | 49.4 | 47.4 | 60.2 | P = 0.075 (not significant) |
CALGB 40601 (n = 299) [20] (WP × 16 plus H and/or L) | 43 | 29 | 52 | Not significant |
CHER-LOB (n = 121) [21] (WP × 12→ FEC × 4 plus H and/or L during whole chemotherapy) | 25 | 26.3 | 46.7 | P = 0.019 |
TRIO-US BO7 (n = 128) [22] TCH × 6 vs. TCL × 6 vs. TCHL × 6 | 47 | 25 | 52 | Not significant |
There are multiple studies that address issue of combined (dual) Her2 therapies as compared to single agent Her2 therapy along with chemotherapy in neoadjuvant setting. Neoadjuvant setting is preferred than adjuvant to study combined blockade because it requires small number of patients, takes months to give results, and correlative scientific studies are applicable with the help of serial biopsies [24]. These studies have been explained further in this article.
Pertuzumab, another HER-2 targeted monoclonal antibody, binds farther from the cell membrane and acts mainly through inhibition of heterodimerization of HER-2 and HER-3. The drug has modest antitumor clinical activity alone but it shows synergism when given along with Trastuzumab [25]. NEOSPHERE neoadjuvant phase 2 study randomized operable Her-2 positive breast cancer patients into one of four arms receiving four cycles of docetaxel plus trastuzumab plus pertuzumab, docetaxel plus trastuzumab, docetaxel plus pertuzumab, and pertuzumab plus trastuzumab without chemotherapy. The pCR were 45.8 % for the combined regimen of docetaxel plus trastuzumab plus pertuzumab, 29 % in docetaxel plus trastuzumab (P = 0.014), 24 % in docetaxel plus pertuzumab (P = 0.003), and 16.8 % in the trastuzumab plus pertuzumab without chemotherapy [26]. Recently, this study has led to FDA approval of the combination of pertuzumab with trastuzumab and chemotherapy as neoadjuvant therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







