200 Splanchnic Ischemia
 Anatomy, Physiology, and Pathophysiology
Anatomy, Physiology, and Pathophysiology
Collateral Circulation
Numerous collaterals may exist or develop. Buhler’s arc contains embryonic remnants of vessels connecting the CA and SMA in the region of the pancreas head and duodenal bulb. Riolan’s artery or marginal artery of Drummond connect the SMA and the IMA. The bowel plexuses also form a large collateral network. Still, even with this large collateral reserve, the superficial layers of the mucosa are very susceptible to the development of ischemia. This susceptibility is due to the countercurrent arteriovenous exchange of oxygen that starts at the base of the villus; when blood flow rate is low, oxygen may be depleted before the villus tip is reached.1–3
Regulation Of Blood Flow
Vasoconstrictors
Catecholamines have different effects on the splanchnic blood flow; α1-adrenergic receptor stimulation leads to vasoconstriction, whereas β2-adrenergic receptor stimulation leads to vasodilatation. The relation between the renin-angiotensin axis and splanchnic perfusion is less uniform, although angiotensin II is a key splanchnic vasoconstrictor during low flow.4 The main splanchnic vasoconstrictor is endothelin (ET)-1.5–6 Two main ET-1 receptor types are have been described: ETA and ETB. Activation of ETA, which is expressed in the mucosa, submucosa, and muscularis of the bowel wall, leads to long-lasting vasoconstriction and plays an important and early role in the negative effects of shock on the integrity of the GI tract.5,7
Vasodilators
The main splanchnic vasodilators are nitric oxide (NO) and prostaglandins. NO has paradoxical effects on gastrointestinal perfusion and mucosal integrity. Normally, low levels of NO are produced by the endothelium to sustain perfusion by promoting local vasodilatation. In pathologic circumstances like circulatory shock or sepsis, a large amount of NO is produced and acts as free radical, similar to oxygen free radicals, and is extremely toxic. In an animal model of hemorrhagic shock, inhibition of NO production is indeed beneficial.8 Locally formed prostaglandins act as mucosal vasodilators, especially during low-flow states or following mucosal injury. Inhibition of cyclooxygenase—for example, with nonsteroidal antiinflammatory drugs (NSAIDs)—diminishes this vasodilatory response and renders the GI mucosa more susceptible to the effects of circulatory shock.9
Low-Flow Conditions
All the above receptors and messengers act to balance perfusion to metabolic demands on a moment-to-moment basis. During circulatory shock, blood flow distribution changes due to constriction and dilatation of different vascular beds. When circulating volume is decreased, relative blood flow to the heart increases and brain perfusion is maintained, but perfusion of skeletal muscles, skin, and gut are reduced. Splanchnic vasoconstriction occurs early and profoundly,10 even before systemic hemodynamic instability arises.11 Splanchnic vasoconstriction can be triggered by different shock states, the direct effects of vasoactive medications, or nicotine and cocaine abuse. GI ischemia occurs only when blood flow is reduced to less than 50% of the basal rate.12–14
During splanchnic hypoperfusion, blood flow within the bowel wall is unevenly distributed among the different layers. In general, the mucosa is protected at the expense of the serosal layers.15 Still, the surface of the mucosa is the most vulnerable area for ischemia, owing to countercurrent diffusional shunting of oxygen. Even within the mucosal layer, blood flow is unevenly distributed. Thus mismatches between metabolic demands and oxygen delivery are caused by several microcirculatory disturbances and shunting.16–18 The patchy distribution of flow when global perfusion is compromised can be observed among different villi as well as within individual villi. These phenomena help explain why, in some studies, mucosal blood flow measurements are within the normal range despite evidence of mucosal ischemia; for early detection of ischemia, flow measurements alone will never suffice. This combination of ischemia despite normal vessel anatomy has given rise to the term NOMI, nonocclusive mesenteric ischemia.
Ischemic Damage
The Ischemic Phase
The immediate effect of reduced oxygen utilization is adenosine triphosphate (ATP) depletion. One of the consequences of ATP depletion is derangements in the tight junctions between adjacent enterocytes, leading to formation of “cracks in the mucosal lining.” Also, key membrane-bound pumps are deprived of energy, and as a consequence, electrolytes and water enter the cells, which swell and, if the process continues, eventually die. Both mechanisms lead to reduced intestinal epithelial barrier function and bacteria moving across the bowel wall from the lumen into the systemic compartment (bacterial translocation).19 During cellular hypoxia, the enzyme, xanthine dehydrogenase, is converted to xanthine oxidase (XO), which is harmless at this stage, because XO needs oxygen as a substrate. Finally, tissue necrosis triggers an inflammatory response, resulting in cytokine release. The effects of the ischemic phase alone are localized and can remain clinically undetected for many hours (closed compartment). The condition sometimes is silent until reperfusion initiates a systemic inflammatory response or transmural gangrene occurs.
Local Effects of Reperfusion
After flow is restored—for example, as a result of the partial dissolution of an embolus—oxygen enters the ischemic tissue. In a reaction catalyzed by XO, oxygen forms reactive oxygen species (ROS) that can damage proteins and DNA.20 The damage to mucosa, blood vessels, and submucosal tissues is not only intensified but spreads to adjacent regions as well by diffusion of the small ROS molecules. Locally present ROS scavengers including glutathione, catalase, and superoxide dismutase, can neutralize ROS, but their efficacy is limited.
Systemic Effects of Reperfusion
Reperfusion delivers toxic products including XO, proinflammatory cytokines, and activated neutrophils into the systemic circulation.21 In animal studies, liver and lung damage have been attributed to activated neutrophils coming from reperfused ischemic bowel.20 Therefore, reperfusion leads to amplification and spreading of the ischemic damage.
 Diagnostic Methods
Diagnostic Methods
Computed Tomography Angiography
CT angiography (CTA), including arterial and venous phase with maximum slice thickness of 1 mm, followed by three-dimensional reconstruction of the vessel anatomy is increasingly used in ICU patients. It has the advantage of minimal invasiveness, very accurate vessel visualization, and additional information on bowel pathology or perfusion. It has recently been reported as an accurate diagnostic test for NOMI. The early introduction of multidetector CT (MDCT) in the decision tree of NOMI treatment, followed by efficient treatment, was safe and suggested to improve mortality.22
Inspection Of The Mucosa And Serosa
With endoscopy, mucosal ischemia can be easily detected; it develops only during malperfusion at a stage where the serosal side is still normal.14 Endoscopy is mostly used to diagnose ischemic colitis after aortic surgery. Endoscopic appearance may be difficult to interpret, especially with imperfectly rinsed bowel; therefore, preparation immediately before endoscopy by enema using 2 to 4 L of water is advisable. Differentiation of ischemic colitis from inflammatory bowel disease can be difficult, and preferably, biopsies should be taken. During the first days, ischemic colitis closely resembles ulcerative colitis; later it may be indistinguishable from Crohn’s disease. Endoscopy cannot distinguish between mucosal and transmural ischemia or gangrene. The latter, irreversible stage can be detected only by inspecting the serosal side of the bowel. Therefore, laparoscopy or laparotomy is indicated when transmural ischemia is suspected.
Laboratory Tests
In general, serologic tests are of limited use for ischemia detection. Classical parameters like leukocyte count and arterial lactate level are of limited value because they lack both sensitivity and specificity. The most promising serologic markers include intestinal fatty acid binding protein (IFABP), D-lactate, ischemia modified albumine, and glutathione S-transferase (GST),23 but clinical data are sparse.24–26
PCO2 Measurement (Tonometry)
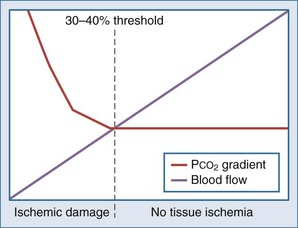
Intraluminal measurement of PCO2 has been shown to detect ischemia, irrespective of flow or metabolism. This extra CO2 is released during ischemia as protons accumulating during anaerobic glycolysis are buffered by tissue bicarbonate. Because CO2 is a small molecule, intraluminal CO2 increases within minutes of increased mucosal CO2. The relationship between CO2 and ischemia was first described in 1979 in heart and skeletal muscles27–28 and in 1982 for the stomach.29 The technique was subsequently popularized by Fiddian-Green and thereafter marketed as tonometry (Figure 200-1, A). He also introduced the term pHi, indicating mucosal acidosis using luminal CO2 and arterial bicarbonate in the Henderson-Hasselbalch equation. Unfortunately, the company making the equipment has decided to stop production, although alternative measurement techniques have been described (see Figure 200-1, B).30–31 Whatever its future, tonometry has demonstrated the important role splanchnic ischemia plays in critical care patients. Moreover, it has enabled us to select patients who could benefit from treatment of splanchnic stenoses.32–34 In the abundance of diagnostics allowing for vessel anatomy assessment, intraluminal PCO2 measurement is the only well-validated test for actual ischemia. An increased intraluminal-to–arterial PCO2 gradient is indicative of ischemia. In the stomach, the normal gastric-arterial PCO2 gradient is below 0.9 kPa (7 mm Hg)35; in the jejunum, the threshold is 1.4 kPa.36 That an increased PCO2 gradient does not relate to changes in perfusion per se can be concluded from studies where the gradient only increases as soon as the splanchnic blood flow decreased to below 30% to 40% of baseline14,35 (Figure 200-2).
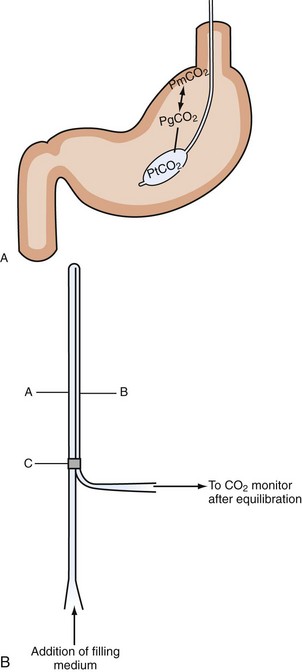
Figure 200-1 Intraluminal PCO2 measurement techniques. A, Tonometry.113 PCO2 can be measured from a specialized balloon-tipped catheter placed in stomach or small or large bowel. Because CO2 diffuses rapidly over different membranes, mucosal PCO2 (PmPCO2) will equal gastric lumen PCO2 (PgCO2). Because the balloon is CO2 permeable as well, balloon PCO2 reflects mucosal values. This balloon PCO2 is measured from air aspirated and inflated automatically into the balloon using a modified capnograph, the Tonocap (Datex-Engström). B, Balloonless intraluminal PCO2 measurement.31 PCO2 is measured using a balloonless catheter, where air flows via a tube which is CO2 permeable only at the intragastrically placed tip and connected with a capnograph on the sampling site.
PCO2 Measurement in the Intensive Care Unit
Because splanchnic ischemia is one of the earliest events in circulatory stress and typically begins at a stage when all other systemic parameters remain within the normal range, it has been referred to as “the canary of the body.”37 Like the canary that was once used in coal mines to detect toxic levels of mine gas, PCO2 measurement may be a good, inexpensive, and relatively early warning of impending trouble.38
Despite its good track record for ischemia detection, PCO2 measurement has not been widely used, either in the ICU or in GI or vascular medicine. Several reasons can be identified for this lack of success. First, saline-based PCO2 measurement was initially laborious, time-consuming, and error prone. Second, many methodological issues clouded the studies in the first years. These included the need for acid suppression and errors introduced by food intake. Third, there was a lack of evidence that tonometry-based ischemia detection led to therapeutic interventions that improved outcome. The first two issues have been properly addressed and resolved by using air-based PCO2 measurement (Tonocap device), potent acid suppression, and use of standardized meals during testing.39–40 Despite its unique properties in the assessment of ischemia, only studies in trauma patients showed an advantage over standard monitoring.41–42 A recent comparative study in septic patients failed to show a survival advantage in patients where resuscitation was aimed at normalization of tonometry, compared to standard systemic parameters.43
Outside the ICU, the situation is different. As a functional test to detect ischemia in the stomach and small bowel, the gold standard is measurement of PCO2 during submaximal exercise, with a 78% sensitivity and 92% specificity.32 Using this exercise test, we could select patients with single-vessel stenosis for treatment and follow-up.34 It enabled us to investigate the entire spectrum of splanchnic stenotic disease from asymptomatic stenoses, to single and multivessel stenoses with ischemic complaints, and finally imminent bowel infarction.44 Measurement of an increased PCO2 after a meal in patients with symptomatic splanchnic stenosis was first shown in 1991.45 Subsequent investigations using gastric PCO2 measurement after a test meal showed variable results,46–47 probably owing to buffering and dilution effects of the test meals.48 With standardized test meals and acid suppression by proton pump inhibitors, the diagnostic accuracy of PCO2 measurement in the stomach and small bowel for detection of ischemia improved considerably.40 Having used this test in over 400 cases, three patterns emerged. First, the normal baseline is below 8 kPa and varies at least 1 kPa. Second, after a liquid meal, the gastric and small-bowel PCO2 did not increase above 10.6 kPa in nonischemic individuals. Third, increased PCO2 levels during the night are quite common and are probably related to buffering effects from duodenogastric reflux. An imminent bowel infarction is characterized by an increased PCO2 for several hours, often above 15 kPa. Also, a suppressed and invariably low PCO2 without the normal variation was seen in patients with an imminent infarction (paper in preparation).
 Clinical Presentations of Splanchnic Ischemia
Clinical Presentations of Splanchnic Ischemia
Splanchnic vascular disorders encompass a spectrum of acute and chronic occlusive, nonocclusive, and aneurysmal disorders affecting the vessels of the abdominal viscera. A classification of ischemic disorders can be made depending on vessel anatomy and ischemia (Figure 200-3). Acute splanchnic ischemia can be caused by arterial embolism, arterial and venous thrombosis, arterial stenoses, or NOMI. For the intensivist, NOMI is the most common problem and will be discussed first. The discussion on occlusive ischemia will focus on the different and often underappreciated clinical presentations, diagnostic problems, and treatment issues, with special emphasis on ICU care.
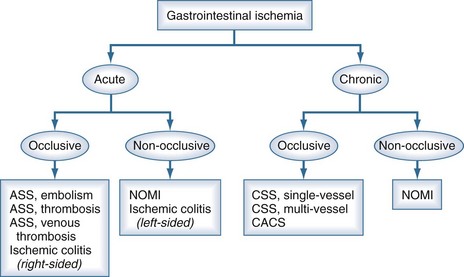
Figure 200-3 Classification of the spectrum of gastrointestinal vascular disease and ischemia.
(Adapted from Kolkman JJ, Bargeman M, Huisman AB, Geelkerken RH. Diagnosis and management of splanchnic ischemia. World J Gastroenterol 2008;14:7309-20.)
Nonocclusive Mesenteric Ischemia
Critically Ill Patients and Major Operations
In gastroenterology and surgery, NOMI is probably a rare disorder that can lead to ischemic colitis49 or acute splanchnic infarction.50 It can also lead to chronic complaints comparable to chronic splanchnic ischemia related to vascular spasm. Treatment with vasodilators has been successful in the majority of patients, and the condition has been referred to as abdominal migraine.51 In many cases, NOMI is reportedly caused by drugs, especially digoxin, or underlying cardiovascular and renal diseases.
NOMI is the end result of the physiologic response to a decreased intravascular blood volume. Early and profound splanchnic vasoconstriction accompanies many major operations, may lead to splanchnic ischemia, and is then associated with an adverse prognosis.52–53 Similarly, in acute pancreatitis, gastric mucosal ischemia was associated with a worse outcome.54 The relevance of this finding was reinforced in a recent randomized study evaluating the effects of probiotics in acute pancreatitis. In this study that investigated the potential beneficial effects of supplementing early feeding with probiotics, the mortality in the probiotic group was significantly higher and was especially associated with bowel infarction.55
It has been suggested that NOMI could play a key role in the pathogenesis of multiple organ failure syndrome (MODS). For example, endotoxinemia can cause mucosal microcirculatory disturbances directly, contributing to hypovolemia-induced vasoconstriction,56 and increased gut-derived cytokine and endotoxin levels have been detected in patients with this syndrome.57–58
Hemodialysis Patients
In hemodialysis patients, NOMI is quite common59 and may lead to bowel infarction in 2%, with a 45% mortality rate.60 The incidence of this complication has been reported in 0.5% to 0.9% of these patients,60–62 in whom NOMI has been associated with hypotension, often during hemodialysis. Close monitoring and prevention of hypotension are crucial to avoid this problem.60
Medications
Many drugs have been implicated as causative agents in NOMI, especially digoxin. NSAIDs affect the integrity of the GI mucus and bicarbonate layer and reduce mucosal perfusion. α-Adrenergic agents like epinephrine and dopamine reduce GI perfusion, and β-adrenergic agents like dobutamine and dopexamine tend to sustain mucosal perfusion.63–65 The clinical importance of these differences is probably very small, because recent comparative studies failed to show differences in mortality between norepinephrine plus dobutamine versus epinephrine,66 and norepinephrine versus dopamine.67
Occlusive Ischemia
The incidence of asymptomatic splanchnic stenoses, so-called chronic splanchnic disease, ranges between 8% and 70% in populations with other manifestations of atherosclerotic disease and is comparable to the incidence of carotid atherosclerosis. Nevertheless, the incidence of symptomatic occlusive splanchnic ischemia, or chronic splanchnic syndrome, is relatively rare, being only 4 to 5 cases per 100,000 inhabitants yearly.68 The incidence of acute splanchnic ischemia is relatively low but increases sharply with age. In a recent autopsy study, it was shown that 1.2% of all deaths in patients over the age of 80 was attributable to acute splanchnic ischemia.69 The diagnosis was suspected in a minority of patients.70
Etiology
External compression by the arcuate ligament of the diaphragm is the predominant cause of single-vessel CA stenosis in young adults. Atherosclerosis is the main cause of single-vessel SMA or IMA occlusive disease and multivessel disease. The latter is defined as stenoses or occlusions in more than one main splanchnic artery. Information on the natural history of splanchnic artery occlusive disease is scarce. Using serial duplex ultrasound, it was demonstrated that visceral artery atherosclerotic stenoses progress in approximately 20% of patients per year. This progression of lesions is especially important in multivessel chronic splanchnic disease, which carries a considerable risk for acute splanchnic infarction.71
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree